The release of the proinflammatory factors is enhanced with increased fat mass, linking adipose tissue inflammation to metabolic dysfunction. Vitamin D3 is a pleiotropic hormone with a range of functions in addition to calcium and bone metabolism. Low vitamin D3 status has been linked to a number of diseases including obesity and metabolic syndrome. Previous studies from our group and others have shown the anti-inflammatory property of 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) in adipose tissue in obesity. However, administration of 1,25(OH)2D3 at a supraphysiological dose can lead to hypercalcemia and this hampers its use as a safe course of treatment. The development of the 1,25(OH)2D3 analogues that have immunomodulatory activity with minimal calcemic effects could be beneficial in clinical practice. ZK159222 and ZK191784 are vitamin D3 analogues known to act on the vitamin D receptors but their effects on adipose tissue particularly the local inflammation are not known. This study investigated whether ZK159222 and ZK191784 are able to modulate the inflammatory response and the signalling pathways involved in human adipocytes. Like 1,25(OH)2D3, treatment with ZK159222 and ZK191784 significantly reduced macrophage-stimulated activation of NF-κB signalling, upregulating IκBα expression and reducing p65 phosphorylation. The phosphorylation of p38 mitogen-activated protein kinase (MAPK) stimulated by macrophage-derived factors was also inhibited by the two compounds. Additionally, macrophage-induced release of the key proinflammatory cytokine/chemokines (IL-6, IL-8, MCP-1 and RANTES) was markedly decreased by the treatment with ZK159222 and ZK191784. Taken together, both 1,25(OH)2D3 analogues have potent anti-inflammatory effects in human adipocytes. These actions are probably mediated by preventing the activation of the NF-κB and MAPK signalling pathways. The data suggests that the two 1,25(OH)2D3 analogues may serve as a potential therapeutic option to have anti-inflammatory effects in adipose tissue in obesity.
ZK159222, ZK191784, 1,25-dihydroxyvitamin D3, NFκB, MAPK, Cytokine, Inflammation, Adipocyte
1,25-dihydroxyvitamin D3 (1,25(OH)2D3), the bioactive form of vitamin D3, is a pleiotropic hormone with a wide range of functions in addition to its classical role in bone and calcium metabolism [1, 2]. It is estimated that vitamin D3 deficiency affects approximately one billion people worldwide [3]. Vitamin D3 deficiency has also been linked to the metabolic disorders, including obesity and insulin resistance [4-6]. Data from clinical studies have shown that low vitamin d3 status are common in obese patients [7-9]. Obesity is now known to be associated with a low-grade chronic inflammation, characterised by a significant increase in accumulation of macrophages and other immune cells in adipose tissue, the activation of inflammatory signalling (i.e. NF-κB and MAPK) pathways and the release of proinflammatory cytokine/chemokines (i.e. TNFα, IL-6, IL-8 and monocyte chemotactic protein-1 (MCP-1)). Adipose tissue inflammation in obesity is considered as a driving force in the pathogenesis of insulin resistance and type 2 diabetes [10-13]. Recent studies from our group and others have demonstrated that 1,25(OH)2D3 has potent anti-inflammatory effects in adipose tissue, such as inhibiting the production of proinflammatory cytokines/chemokines by preadipocytes and adipocytes, and reducing macrophage infiltration [14-17]. A main concern of using 1,25(OH)2D3 for treatment is the possible disruption of calcium homeostasis inducing hypercalcemia [18,19]. Furthermore, circulating levels of 1,25(OH)2D3 has been shown to be negatively correlated with coronary calcification [20]. 1,25(OH)2D3 is known to exert a wide range of effects, which can be classified as genomic and non-genomic. The genomic effects of 1,25(OH)2D3 is characterised by its binding to the vitamin D receptor (VDR) in the cytoplasm, nuclear translocation of the liganded receptor and interaction with vitamin D response elements in the promoter region of genes, leading to changes in gene expression within hours [21]. Its non-genomic effects include rapid changes in intracellular calcium concentrations, membrane phospholipid metabolism and activation of protein kinases, which is involved in several signal transduction cascades, and the non-genomic effects of 1,25(OH)2D3 may be VDR independent [22-25]. Despite the potential therapeutic effects of 1,25(OH)2D3 in vitro, its clinical use could be limited mostly due to unwanted hypercalcemic effects.
The development of 1,25(OH)2D3 analogues, with low calcemic effects but selective actions on VDR-mediated gene transcription, may offer an attractive solution in clinical practice. ZK159222 and ZK191784 are the 1,25(OH)2D3 analogues, both of which act on the vitamin D receptors [26,27]. ZK159222 is a partial antagonist of VDR, characteristised by a long butyl ester at the C25 position of the side chain [26]. In contrast to the natural hormone, ZK159222 appears to prevent the interaction of VDR with coactivators [28]. However, like 1,25(OH)2D3, the binding of ZK159222 to the VDR can induce a dissociation of the majority of the VDR-corepressor complexes [29]. These observations suggest that the antagonistic quality of ZK159222 is achieved by stabilising the VDR in a conformation that blocks coactivator to interact with vitamin D response elements (VDREs), without affecting the normal VDR-corepressor dissociation (Toell, Gonzalez et al. 2001) [28]. It has been shown that ZK159222 is not a complete antagonist as it still retains approximately 20% of the agonistic ability of 1,25(OH)2D3 [28,30]. However, whether ZH159222 has immunomodulatory activity is largely unknown.
ZK191784 is characterised by an altered side chain structure containing a 22,23-double bond, 24R-hydroxy group, 25-cyclopropyl ring, and 5-butyloxazole-group [27]. ZK191784 has been reported to competitively binds to the VDR with a similar affinity as 1,25(OH)2D3 [31]. Unlike 1,25(OH)2D3, ZK191784 dose not stimulate intestinal Ca2+ absorption and reduces renal Ca2+ excretion that it has less calcemic effects than 1,25(OH)2D3 [27]. Studies in vivo and in vitro have suggested that ZK191784 exhibit immunomodulatory activity. ZK191784 has been shown to have therapeutic potential in T cell-mediated immune disorders with a 100-fold lower hypercalcemic effect in comparison with 1,25(OH)2D3 in rats [31]. In experimental colitis in mice, ZK191784 treatment ameliorated acute and chronic intestinal inflammation probably by its immunosuppressive effects on mucosal dendritic cells without exhibiting calcemic effects [32]. Moreover, ZK191784 downregulated adhesion molecules and MMPs production in colonic biopsies of patient with inflammatory bowel diseases [33].
Although ZK159222 and ZK191784 have congruent effects on the VDR, their roles in adipose tissue particularly the local inflammation which often occurs in obesity are not known. This study was therefore to investigate whether the two 1,25(OH)2D3 analogues modulate the inflammatory responses and the signalling pathways involved in human adipocytes. Their effects were compared to the action of the natural ligand, 1,25(OH)2D3.
Culture of adipocytes
Human preadipocytes derived from subcutaneous adipose tissue of a female Caucasian subject (BMI 21 kg/m2; age 44 yr) were obtained from PromoCell (Heidelberg, Germany). Cells were seeded at 40,000/cm2 and grown in 24-well plates in preadipocyte growth medium, containing DMEM-Ham’s F-12 (1:1, vol/vol) and supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B (Lonza, Twekesbury, UK), at 37°C in a humidified atmosphere of O2:CO2 (95:5%). At confluence, cells were induced to differentiate at day 0 by incubation for 3 days in Dulbecco's Modified Eagle's Medium (DMEM) and Ham's F12 (1:1 ratio) medium which contained 32 μM biotin, 1μM dexamethasone, 200 μM 3-isobutyl-1-methyl-xanthine, 100 nM insulin, 11 nM L-Thyroxine (all from Sigma, Poole, Dorset, UK), 8 μM rosiglitazone (GlaxoSmithKline, Uxbridge, UK), 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B. After induction, cells were cultured in maintenance medium containing 3% foetal calf serum (FCS; Sigma), 100 nM insulin, 32 μM biotin and 1 μM dexamethasone until fully differentiated. Differentiation into mature adipocytes was confirmed by observing the accumulation of lipid droplets under the microscope.
Culture of THP-1 macrophages
Human THP-1 myelomonocytic cell line (Health Protection Agency Culture Collections, Porton Down, Salisbury UK) was used. THP-1 monocytes (1×106 cells/ml) were cultured in a 150 cm2 flask in Roswell Park Memorial Institute (RPMI-1640) medium, containing 10% FCS, 100 U/ml penicillin and 100 μg/ml streptomycin, at 37°C in a humidified atmosphere of O2:CO2 (95:5%). THP-1 monocytes were differentiated into macrophages with 100 nM phorbol 12-myristate 13-acetate (PMA) (Sigma) for 48 h. The medium was replaced with PMA-free and FCS-free RPMI-1640 medium for 24 h. This medium as macrophage-conditioned (MC) medium was collected, filtered through a 0.22 µm filter and stored at -80°C for later use.
Cell treatment
ZK159222 and ZK191784, VDR ligands, were a generous gift from Bayer Pharma AG (Berlin, Germany) [31, 34, 35]. Differentiated adipocytes (Day 11) were grouped and incubated with adipocytes maintenance media, 10-8M of 1,25(OH)2D3 (Sigma) , ZK159222 (10-8M or 10-6M) and ZK191784 (10-8M or 10-6M) for 48 h. The cell culture medium was replaced with RPMI (control), MC medium (MC), MC medium with 1,25(OH)2D3 (10-8M), MC medium with ZK159222 (10-8M or 10-6M) and MC medium with ZK191784 (10-8M or 10-6M) for 24 h. The culture medium and cell lysates were collected and stored at -80°C until later analyses.
Western blotting
Cell lysates were prepared by lysing cells in lysis buffer (50 mM Tris-HCl pH 6.7, 10% glycerol, 4% SDS, 2% 2-mer-capoethanol) with protease inhibitor cocktail and phosphatase inhibitors (Sigma). Protein concentrations of the lysates were then measured by the bicinchoninic acid method. Protein samples were separated on 10% Tricine-SDS polyacrylamide gels (Mini Protean Tetra, Bio-Rad, Hemel Hempstead, UK) and transferred to nitrocellulose membranes at 100v for 1 h. Protein marker was used on one lane for identification of target bands. The membranes were blocked with 5% BSA in Tris-buffered saline (TBS) and 0.1% Tween-20 for 1 h at room temperature. The membranes were then incubated with the primary antibody, including Ικβα, phosphorylated NFκB p65 and phosphorylated p38 MAPK (all from New England Biolabs, Hitchin, UK) overnight at 1:1000 dilution at 4°C. The membranes were washed in phosphate-buffered saline with 0.1% Tween-20 and subsequently incubated with a HRP-conjugated secondary antibody (New England Biolabs). Chemiluminescent signals were evaluated and quantified using a SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL, US) using the ChemiDoc XRS+ System (Bio-Rad). The membranes were further probed for vinculin or GAPDH (both from Abcam, Cambridge, UK) as a loading control.
Measurement of cytokine/chemokine release
The release of IL-6, IL-8, MCP-1 and RANTES by adipocytes and THP-1 macrophages was measured as protein concentrations in culture medium, using the DuoSet ELISA Development kits (R&D Systems, Abingdon, UK).
Cytotoxicity evaluation
Adipocyte viability after treatment with various compounds was assessed as release of lactate dehydrogenase (LDH) into the cell culture medium, using a colourimetric cytotoxicity detection kit (Roche Diagnostics GmbH, Mannheim, Germany). LDH levels were measured at room temperature by a spectrophotometer at 492 nm with a reference wavelength of 620 nm.
Statistical analysis
All results are presented as means ± standard error of mean (SEM). Statistical differences among groups were assessed by one-way ANOVA coupled with Bonferroni’s t-test. Differences were considered as statistically significant when P value <0.05. Statistical tests were performed using Graphpad Prism version 5.03 and SPSS(PASW), release version 19.0.
ZK 159222 and ZK191784 inhibit macrophage-induced activation of NF-κB in human adipocytes.
This set of experiments examined whether ZK159222 and ZK191784 have similar effects to 1,25-dihydroxyvitamin D3 on macrophage-induced NF-κB activation in adipocytes. As shown by Figure 1, treatment with 1,25(OH)2D3 led to an increase in IκBα expression (P = 0.003) in adipocytes stimulated with MC medium. ZK159222 treatment reversed MC medium-induced down regulation of IκBα in a dose-dependent manner, inducing a 3.9-fold at 10-8M although this was not statistically significant (P = 0.148) and a 7.4-fold increase at 10-6M (P = 0.002). Compared to 1,25(OH)2D3, the effect of ZK159222 at 10-8M was less potent but it had a similar effect to 10-8M 1,25(OH)2D3 at 10-6M.
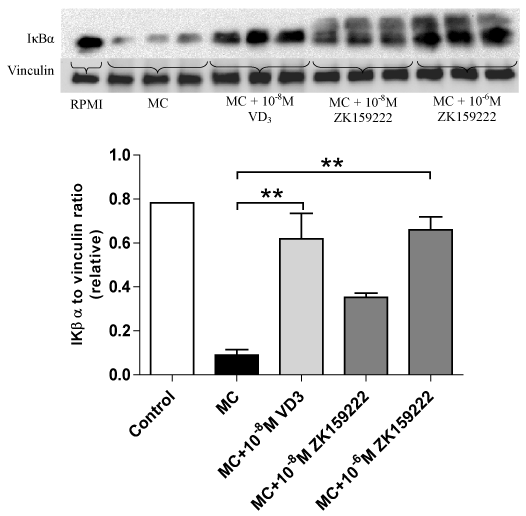
Figure 1. ZK159222 reverses macrophage-inhibited IκBα expression by adipocytes. Differentiated adipocytes were incubated with maintenance medium, 1,25-(OH)2D3 (10-8M) or ZK159222 (10-8M and 10-6M) for 48 h. Adipocyte medium was then replaced with RPMI-1640 medium (control), MC medium or MC medium with 1,25-(OH)2D3 (10-8M) or ZK159222 (10-8M and 10-6M) for 24 h. IκBα protein content was determined by western blotting and quantified by densitometry. Data are means ± SEM, normalised to vinculin levels, n=3 per group. **P<0.01 vs. MC group.
ZK159222 also downregulated the induction of phosphorylated NF-κB p65 expression by MC medium. While 1,25(OH)2D3 (10-8M) decreased phosphorylated NF-κB p65 by 67% (P = 0.011), ZK159222 significantly reversed MC medium-induced upregulation of phosphorylated NF-κB p65 by 90% at 10-8M (P = 0.002) and 91% at 10-6M (P = 0.001) compared to adipocytes that received treatment with MC medium only. ZK159222 appeared to have a greater effect in reducing phosphorylated NF-κB p65 compared with 1,25(OH)2D3, although there were no statistically significant differences among three groups that received treatment with 1,25(OH)2D3 or its analogues (Figure 2).
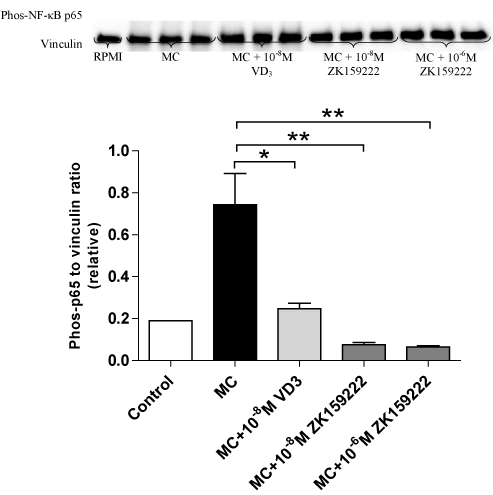
Figure 2. ZK159222 inhibits macrophage-induced expression of NF-κB p65 by adipocytes. Differentiated adipocytes were incubated with maintenance medium, 1,25-(OH)2D3 (10-8M) or ZK159222 (10-8M and 10-6M) for 48 h. Adipocyte medium was then replaced with RPMI-1640 medium (control), MC medium or MC medium with 1,25-(OH)2D3 (10-8M) or ZK159222 (10-8M and 10-6M) for 24 h. Phos-NF-κB p65 protein content was analysed by western blotting and quantified by densitometry. Data are means ± SEM, normalised to vinculin levels, n=3 per group. *P<0.05, **P<0.01 vs. MC group.
As shown by Figure 3, ZK191784, another analogue of 1,25(OH)2D3, displayed similar effects as 1,25(OH)2D3. Like 1,25(OH)2D3 which elicited a 9.1-fold increase in IκBα expression (P = 0.004), ZK191784 treatment dose-dependently reversed macrophage-induced downregulation of IκBα, leading to a 5.4-fold increase at 10-8M although this was not statistically significant (P = 0.11), and a 10-fold increment at 10-6M (P = 0.002). There were no statistically significant differences among three groups that received treatment with vitamin D3 or its analogues.
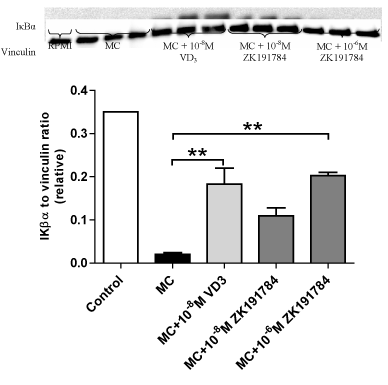
Figure 3. Effects of ZK191784 on macrophage-inhibited expression of IκBα by adipocytes. Differentiated adipocytes were incubated with maintenance medium, 1,25-(OH)2D3 (10-8M) or ZK191784 (10-8M and 10-6M) for 48 h. Adipocyte medium was replaced with RPMI-1640 medium (control), MC medium or MC medium with 1,25-(OH)2D3 (10-8M) or ZK191784 (10-8M and 10-6M) for 24 h. IκBα protein content was analysed by western blotting and quantified by densitometry. Data are means ± SEM, normalised to vinculin levels, n=3 per group. **P<0.01 vs. MC group.
Similar to 1,25(OH)2D3, ZK191784 also downregulated protein expression of phosphorylated NF-κB p65 in adipocytes stimulated with MC medium (Figure 4). While 1,25(OH)2D3 decreased phosphorylated NF-κB p65 by 41% (P<0.001), ZK191784 reduced phosphorylated NF-κB p65 by 57% at 10-8M (P<0.001) and 59% at 10-6M (P<0.001) compared with adipocytes that received treatment with MC medium only. Compared to 1,25(OH)2D3 (10-8M), ZK191784 showed a greater potency in reducing phosphorylated NF-κB p65 at both concentrations (P = 0.02 for 10-8M, P = 0.009 for 10-6M).
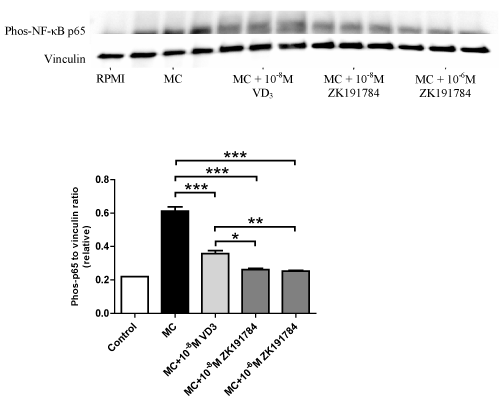
Figure 4. Effects of ZK191784 on macrophage-induced expression of NF-κB p65 by adipocytes. Differentiated adipocytes were incubated with maintenance medium, 1,25-(OH)2D3 (10-8M) or ZK191784 (10-8M and 10-6M) for 48 h. Adipocyte medium was replaced with RPMI-1640 medium (control), MC medium or MC medium with 1,25-(OH)2D3 (10-8M) or ZK191784 (10-8M and 10-6M) for 24 h. Phos-NF-κB p65 protein content was analysed by western blotting and quantified by densitometry. Data are means ± SEM, normalised to vinculin levels, n=3 per group. **P<0.05, **P<0.01, ***P<0.001 vs. MC group.
ZK 159222 and ZK191784 inhibit macrophage-induced expression of p38 MAPK in human adipocytes
Since both vitamin D3 compounds modulate the activation of NF-κB, we next examined whether ZK159222 and ZK191784 have effects on the MAPK signalling pathway in adipocytes. As shown by Figure 5A, similar to 1,25(OH)2D3 which caused a 42% decrease in phosphorylated p38 MAPK (P = 0.002), ZK159222 partially reversed macrophage-induced upregulation of phosphorylated p38 MAPK in a dose-dependent manner, with a 27% decrease at 10-8M (P = 0.03) and a 77% decrease at 10-6M (P < 0.001). Compared to 1,25-dihydroxyvitamin D3, the level of reduction in phosphorylated p38 MAPK by ZK159222 was not significantly different at 10-8M (P = 0.45) but the inhibitory effect was enhanced at 10-6M (P=0.006).
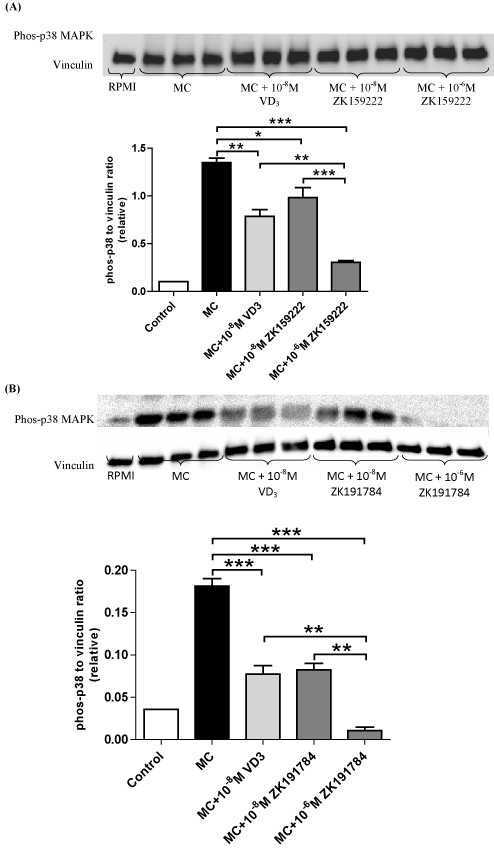
Figure 5. ZK159222 and ZK191784 inhibit macrophage-induced expression of Phos-p38 MAPK by adipocytes. Differentiated adipocytes were incubated with maintenance medium, 1,25-(OH)2D3 (10-8M) or ZK191784 (10-8M and 10-6M) for 48 h. Adipocyte medium was replaced with RPMI-1640 medium (control), MC medium or MC medium with 1,25-(OH)2D3 (10-8M), ZK159222 (10-8M and 10-6M), ZK191784 (10-8M and 10-6M) for 24 h. Effect of ZK159222 (A) and ZK191784 (B) on phos-NF-κB p65 protein content was analysed using western blotting and quantified by densitometry. Data are means ± SEM, normalised to vinculin levels, n=3 per group. **P<0.01, ***P<0.001 vs. MC group.
Similar to 1,25(OH)2D3, ZK191784 downregulated phosphorylated p38 MAPK expression in adipocytes stimulated with MC medium (Figure 5B). While 1,25(OH)2D3 decreased phosphorylated p38 MAPK by 57% (P<0.001), ZK191784 dose-dependently reversed upregulation of phosphorylated p38 MAPK by MC medium, inducing a 55% decrease at 10-8M (P<0.001) and a 94% at 10-6M (P<0.001). At similar concentrations (10-8M), there was no significant difference in the potency of inhibition on phosphorylated p38 MAPK between ZK191784 and 1,25(OH)2D3 (P>0.05) but at higher dose (10-6M) the inhibitory effect of ZK191784 was greater (P = 0.002).
ZK159222 and ZK191784 inhibit macrophage-induced secretion of IL-6, MCP-1, IL-8 and RANTES by human adipocytes
To further study the downstream effects of inhibiting NF-κB and MAPK signalling by ZK159222 and ZK191784, we examined the release of some key cytokine and chemokines in adipocytes. As shown by Figure 6, MC medium-induced IL-6 secretion by adipocytes was reduced 36% following the treatment with 1,25(OH)2D3 compared to adipocytes incubated in MC medium alone (P < 0.001). 10-8M and 10-6M of ZK159222 reduced macrophage-induced IL-6 release by 52% and 71% respectively (both P < 0.001). 10-8M and 10-6M of ZK191784 reduced IL-6 release from adipocytes by 35% and 54% respectively (both P < 0.001). Furthermore, the inhibitory effect of 10-6M ZK159222 on IL-6 production was stronger compared with 10-8M of 1,25(OH)2D3 (54% decrease, P < 0.001).
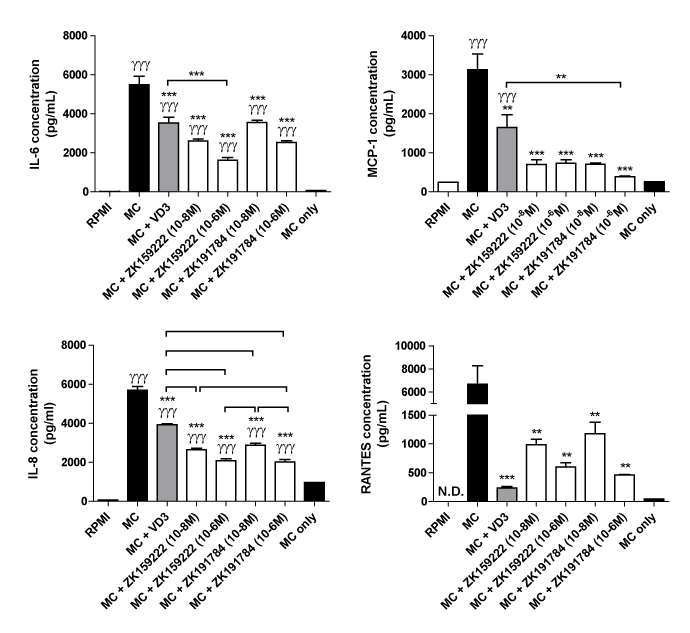
Figure 6. Effect of ZK159222 and ZK191784 on macrophage-induced secretion of IL-6, MCP-1, IL-8 and RANTES by human adipocytes. Differentiated adipocytes were incubated with maintenance medium, 1,25-(OH)2D3 (10-8M) or ZK191784 (10-8M and 10-6M) for 48 h. Adipocyte medium was replaced with RPMI-1640 medium (control), MC medium or MC medium with 1,25-(OH)2D3 (10-8M), ZK159222 (10-8M and 10-6M), ZK191784 (10-8M and 10-6M) for 24 h. Protein release of IL-6, MCP-1 , IL-8 and RANTES was measured as protein concentrations in the culture medium using ELISAs. Results are expressed as means ± SEM, n=3 per group. γγγP<0.001 vs. RPMI control; *P<0.05, **P<0.01, ***P<0.001 vs. MC control and ††P<0.01, †††P<0.001 vs. MC + 1,25-dihydroxyvitamin D3. N.D. – not detected. MC only, macrophage-conditioned medium was used for measuring basal levels of the cytokine/chemokines released by macrophages.
In Figure 6, MC medium-induced MCP-1 secretion by adipocytes was reduced 47% with the treatment of 1,25(OH)2D3 compared to adipocytes incubated in MC medium alone (P = 0.001). MCP-1 release was reduced by 78% and 77% by adipocytes treated with 10-8M and 10-6M of ZK159222 (both P < 0.001). Similarly, MCP-1 release by adipocytes treated with 10-8M and 10-6M of ZK191784 was reduced by 77% and 88% respectively (both P < 0.001). Of the compounds tested, only 10-6M of ZK191784 showed a greater effect in lowering MCP-1 secretion compared with 10-8M 1,25(OH)2D3 (P < 0.01).
Furthermore, MC medium-stimulated IL-8 secretion by adipocytes was reduced by 31% with the treatment of 1,25(OH)2D3 (P<0.001). Treatment with 10-8M and 10-6M of ZK159222 reduced IL-8 release by 53% and 64% respectively (both P<0.001). Treatment with 10-8M and 10-6M of ZK191784 reduced IL-8 release by 49% and 65% in a dose-dependent manner (both P<0.001). Compared with 10-8M of 1,25(OH)2D3, IL-8 secretion was significantly lower from adipocytes treated with ZK159222 and ZK191784 at all doses (P < 0.001).
Finally, MC medium-induced RANTES secretion by adipocytes treated with 1,25(OH)2D3 was attenuated (by 96%, P<0.001). Treatment with 10-8M and 10-6M of ZK159222 markedly reduced macrophage-induced release of RANTES (by 85% and 91% respectively, both P = 0.002). Similarly, treatment with 10-8M and 10-6M of ZK191784 significantly reduced RANTES release by adipocytes by 82% (P=0.004) and 93% (P=0.005), respectively. Compared with 10-8M of 1,25(OH)2D3, there were no significant differences in RANTES secretion by ZK159222 and ZK191784 treatment at all doses.
Effects of MC medium, 1,25-dihydroxyvitamin D3, ZK159222 and ZK191784 on cell viability
Cytotoxicity of the compounds (MC medium, 1,25(OH)2D3, ZK159222 and ZK191784) used in this study were examined by the measurement of LDH release into cell culture media. As shown by Figure 7, there was no significant difference between the control and the treatment groups.
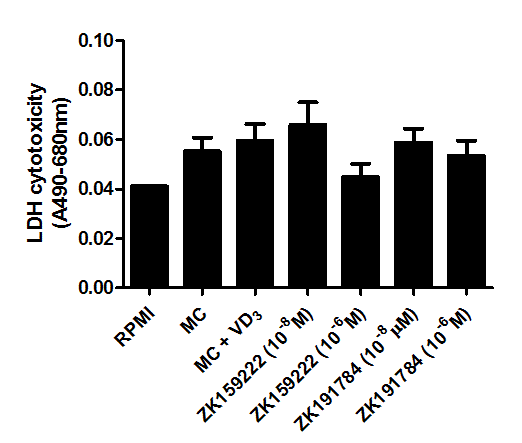
Figure 7. Cytotoxicity of macrophage-conditioned medium, 1,25-(OH)2D3, ZK159222 and ZK191784 in adipocytes. Differentiated adipocytes were incubated with maintenance medium, 1,25-(OH)2D3 (10-8M) or ZK191784 (10-8M and 10-6M) for 48 h. Adipocyte medium was replaced with RPMI-1640 medium (control), MC medium or MC medium with 1,25-(OH)2D3 (10-8M), ZK159222 (10-8M and 10-6M), ZK191784 (10-8M and 10-6M) for 24 h. Cytotoxicity was determined as LDH release into the cell culture medium. Data are expressed as means ± SEM, n=3 per group.
Chronic inflammation has an important role in the pathogenesis of obesity and its related metabolic disorders [36,37]. Adipose tissue as a major endocrine organ releases a wide range of protein factors, including cytokines/chemokines (i.e., IL-1, IL-6, IL-8 and MCP-1) [38]. The release of the proinflammatory factors is enhanced with increased fat mass, linking adipose tissue inflammation to metabolic dysfunction [39]. Previous studies from our group and others have provided evidence for the anti-inflammatory property of 1,25(OH)2D3 in adipose tissue, which was demonstrated by reduced proinflammatory response in adipocytes stimulated by macrophage-derived factors, cytokines and LPS as well as in adipose tissue of obese mice induced by high-fat diet [14,16,17,40,41]. However, these studies suggest that supraphysiological doses of 1,25(OH)2D3 are required to have a significant effect in adipose tissue. As using high doses of 1,25(OH)2D3 has been linked to the increased risk of hypercalcemic side effects, it is unlikely that 1,25(OH)2D3 can be used as a sustainable treatment particularly for those with renal insufficiency [42, 27]. The analogues of 1,25(OH)2D3 with low calcemic effects may offer an alternative solution in clinical practice. A number of 1,25(OH)2D3 analogues have been developed with the purpose of improving the biological profile of the natural hormone for potential therapeutic value [26]. The current study therefore investigated whether the two 1,25(OH)2D3 analogues, ZK159222 and ZK191784, have modulatory effects on adipose tissue inflammation in vitro.
In the present study, we demonstrate for the first time the anti-inflammatory actions of the two 1,25(OH)2D3 analogues in human adipocytes. Our study initially examined the effects of ZK159222 and ZK191784 on the NF-κB signalling pathway. The transcription factor NF-κB is a key regulator of inflammatory gene expression and its activity is enhanced in adipose tissue during obesity [43, 44]. NF-κB is activated by the degradation of IκBα protein, allowing nuclear translocation of the p65 subunit of NF-κB [45]. In the current study, ZK159222 was able to reverse IκBα expression suppressed by MC medium in adipocytes although to a lesser extent when compared to 1,25(OH)2D3 in equimolar concentrations. In addition, both doses of ZK159222 (10-8M and 10-6M) inhibited macrophage-induced phosphorylation of NF-κB p65 and this effect appeared to be stronger in comparison with 1,25(OH)2D3 (Figure 2). Although ZK159222 is an antagonist of VDR being used to study the effect of full VDR activation by 1,25(OH)2D3, it still has some agonistic potential of 1,25(OH)2D3 [46, 30]. However, little is known whether ZK159222 has immunomodulatory property. A previous study has shown that ZK159222 inhibited 1,25(OH)2D3-induced NO production in porcine aortic endothelial cells [47]. In the present study, ZK159222 enhanced IκBα stability supporting the notion that VDR binding stabilises IκBα in adipocytes. Furthermore, NF-κB p65 phosphorylation has been shown to be mediated by intracellular calcium levels and 1,25(OH)2D3 is also a non-genomic enhancer [48, 49]. As ZK159222 is not known to have non-genomic effect, it may have greater potency in preventing the phosphorylation of NF-κB p65. In the present study, we have also shown that ZK191784 can reverse IκBα expression inhibited by macrophage-derived factors in adipocytes. Moreover, ZK191784 at both doses (10-8M and 10-6M) attenuated upregulation of phosphorylated NF-κB p65 stimulated by MC medium, and this effect was stronger than 1,25(OH)2D3 (Figure 4). Taken together, our data suggest that ZK159222 and ZK191784 have strong inhibitory effects on macrophage-induced activation of NF-κB signalling in human adipocytes.
The activation of the MAPK signalling is also involved in the signal transduction of inflammatory mediators leading to inflammatory response in several cell types including preadipocytes and adipocytes [16, 50, 51]. In this study, we have shown that ZK159222 and ZK191784 are able to inhibit the activation of the MAPK signalling in human adipocytes. Both 1,25(OH)2D3 analogues inhibited phosphorylation of p38 MAPK in a dose-dependent manner. Moreover, their inhibitory effect was comparable to that of 1,25(OH)2D3 (Figure 5). Therefore, the MAPK signalling pathway could be potential target of the 1,25(OH)2D3 analogues for reducing inflammatory response in adipose tissue.
To further study the downstream effects of ZK159222 and ZK191784 on adipose tissue inflammation, we have examined the release of the key proinflammatory cytokine and chemokines by adipocytes upon macrophage stimulation. IL-6, a major cytokine and expressed by adipocytes, is critically involved in obesity related inflammation and insulin resistance [52, 53]. In the present study, ZK159222 and ZK191784 markedly reduced protein release of IL-6 by adipocytes as the natural hormone 1,25(OH)2D3 (Figure 6). Although the effect of the two compounds on IL-6 release was unknown previously, ZK191784 has been shown to inhibit LPS-induced secretion of cytokine IL-12 and TNF-α by human PBMCs, with a similar potency as 1,25(OH)2D3 [31]. MCP-1 is a chemokine with a chemotactic activity for monocytes and its overexpression enhances macrophage infiltration and insulin resistance [54, 55]. The current study has shown that ZK159222 and ZK191784 markedly reduced MCP-1 secretion (77-88%) elicited by MC medium to an extent similar to the treatment with 1,25(OH)2D3. Moreover, the higher dose (10-6M) of ZK191784 had strongest inhibitory effect on MCP-1 release by adipocytes (Figure 6). IL-8 as a neutrophil chemotactic factor has been shown to induce chemotaxis in other cell types including macrophages, and it has been reported that lack of IL-8 receptor reduced macrophage infiltration in adipose tissue [56, 57]. In the present study, ZK159222 and ZK191784 decreased macrophage-induced IL-8 release (up to 65%), with a greater efficacy compared to 1,25(OH)2D3. Chemokine RANTES is another key player in obesity-associated inflammation in adipose tissue. RANTES stimulates monocyte migration and macrophage survival in human adipose tissue; its expression in adipose tissue is increased in obese patients [58]. Like 1,25(OH)2D3, both analogues significantly reduced macrophage-induced RANTES secretion (82-93%) by adipocytes. Taken together, our results show that ZK159222 and ZK191784 produced comparable effects to 1,25(OH)2D3 in reducing the production of the cytokine/chemokines by adipocytes. It is suggested that both compounds have anti-inflammatory actions similar to the natural ligand in adipose tissue.
In conclusion, we have demonstrated that ZK159222 and ZK191784 possess anti-inflammatory properties in adipose tissue. Both 1,25(OH)2D3 analogues powerfully inhibit macrophage-induced secretion of the proinflammatory cytokine and chemokines by adipocytes. This effect is probably via preventing the activation of the NF-κB and MAPK signalling pathways. Our study suggests that the 1,25(OH)2D3 analogues may serve as a potential option to enhance VDR-mediated anti-inflammatory effects in adipose tissue in obesity.
We would like to thank Dr Arndt Schmitz (Bayer Pharma AG) for providing ZK159222 and ZK191784. This study was financially supported by the University of Liverpool.
- Boyle IT, Miravet L, Gray RW, Holick FW, Deluca HF (1972) Response of intestinal calcium transport to 25-hydroxy and 1,25-dihydroxy vitamin-d in nephrectomized rats. Endocrinology 90: 605. [Crossref]
- Mellanby E (1976) Nutrition classics. The lancet 1:407-12, 1919. An experimental investigation of rickets. Edward mellanby. Nutr Rev 34: 338-340. [Crossref]
- Holick MF (2007) Vitamin d deficiency. New England Journal of Medicine 357(3): 266-281. [Crossref]
- Chiu KC, Chu A, Go VL, Saad MF (2004) Hypovitaminosis d is associated with insulin resistance and beta cell dysfunction Am J Clin Nutr 79: 820-825. [Crossref]
- Barchetta I, Angelico F, Del Ben M, Baroni MG, Pozzilli P, et al (2011) Strong association between non-alcoholic fatty liver disease (nafld) and low 25(oh) vitamin d levels in an adult population with normal serum liver enzymes. BMC Med 9: 85. [Crossref]
- Olson ML, Maalouf NM, Oden JD, White PC, Hutchison MR (2012) Vitamin d deficiency in obese children and its relationship to glucose homeostasis. J Clin Endocrinol Metab 97: 279-285. [Crossref]
- Goldner WS, Stoner JA, Thompson J, Taylor K, Larson L, et al (2008) Prevalence of vitamin d insufficiency and deficiency in morbidly obese patients: a comparison with non-obese controls. Obes Surg 18: 145-150. [Crossref]
- Brock K, Huang WY, Fraser DR, Ke L, Tseng M, et al (2010) Low vitamin d status is associated with physical inactivity, obesity and low vitamin d intake in a large us sample of healthy middle-aged men and women. J Steroid Biochem Mol Biol 121: 462-466. [Crossref]
- Fish E, Beverstein G, Olson D, Reinhardt S, Garren M, et al. (2010) Vitamin d status of morbidly obese bariatric surgery patients. J Surg Res 164: 198-202. [Crossref]
- Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S (2007) Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 56: 1010-1013. [Crossref]
- Bourlier V and Bouloumie A (2009) Role of macrophage tissue infiltration in obesity and insulin resistance. Diabetes Metab 35: 251-260. [Crossref]
- Lolmede K, Duffaut 2021 Copyright OAT. All rights reserv; A (2011) Immune cells in adipose tissue: key players in metabolic disorders. Diabetes Metab 37: 283-290. [Crossref]
- Gao D, Madi M, Ding C, Fok M, Steele T, et al. (2014). Interleukin-1beta mediates macrophage-induced impairment of insulin signaling in human primary adipocytes. Am J Physiol Endocrinol Metab 307: e289-304. [Crossref]
- Lorente-cebrian S, Eriksson A, Dunlop T, Mejhert N, Dahlman I, et al (2012) Differential effects of 1alpha,25-dihydroxycholecalciferol on mcp-1 and adiponectin production in human white adipocytes. Eur J Nutr 51: 335-342. [Crossref]
- Marcotorchino J, Gouranton E, Romier B, Tourniaire F, Astier J, et al (2012) Vitamin d reduces the inflammatory response and restores glucose uptake in adipocytes. Mol Nutr Food Res 56:1771-1782 [Crossref]
- Ding C, Wilding JP, Bing C (2013) 1,25-dihydroxyvitamin D3 protects against macrophage-induced activation of nfkappab and mapk signalling and chemokine release in human adipocytes. Plos One 8: e61707. [Crossref]
- Gao D, Trayhurn P, Bing C (2013) 1,25-dihydroxyvitamin d3 inhibits the cytokine-induced secretion of mcp-1 and reduces monocyte recruitment by human preadipocytes. Int J Obes (lond) 37: 357-365. [Crossref]
- Smith DC, Johnson CS, Freeman CC, Muindi J, Wilson JW, et al (1999) A phase i trial of calcitriol (1,25-dihydroxycholecalciferol) in patients with advanced malignancy. Clinical Cancer Research 5: 1339-1345. [Crossref]
- Slatopolsky E, Brown AJ (2002) Vitamin d analogs for the treatment of secondary hyperparathyroidism. Blood Purification 20: 109-112. [Crossref]
- Watson KE, Abrolat ML, Malone LL, Hoeg JM, Doherty T, et al (1997) Active serum vitamin d levels are inversely correlated with coronary calcification. Circulation 96: 1755-1760. [Crossref]
- Haussler MR, Jurutka PW, Mizwicki M, Norman AW (2011) Vitamin d receptor (vdr)-mediated actions of 1 alpha,25(oh)(2)vitarnin d-3: genomic and non-genomic mechanisms. Best Practice & Research Clinical Endocrinology & Metabolism 25: 543-559. [Crossref]
- Kajikawa M, Ishida H, Fujimoto S, Mukai E, Nishimura M, et al (1999) An insulinotropic effect of vitamin d analog with increasing intracellular Ca2+ concentration in pancreatic beta-cells through nongenomic signal transduction. Endocrinology 140: 4706-4712. [Crossref]
- Mizwicki MT, Norman AW (2009) The vitamin d sterol-vitamin d receptor ensemble model offers unique insights into both genomic and rapid-response signaling. Science Signaling 2. [Crossref]
- Menegaz D, Barrientos-duran A, Kline A, Silva FR, Norman AW, et al (2010) 1 alpha,25(oh)(2)-vitamin d-3 stimulation of secretion via chloride channel activation in sertoli cells. Journal of Steroid Biochemistry and Molecular Biology 119: 127-134. [Crosssref]
- Hii CS, Ferrante A (2016) The non-genomic actions of vitamin d. Nutrients 8. [Crossref]
- Carlberg C (2003) Molecular basis of the selective activity of vitamin d analogues. Journal of Cellular Biochemistry 88: 274-281. [Crossref]
- Nijenhuis T, Van der eerden BC, Zügel U, Steinmeyer A, Weinans H, et al (2006) The novel vitamin d analog zk191784 as an intestine-specific vitamin d antagonist. Faseb J 20: 2171-2173. [Crossref]
- Toell A, Gonzalez MM, Ruf D, Steinmeyer A, Ishizuka S, et al (2001) Different molecular mechanisms of vitamin d-3 receptor antagonists. Molecular Pharmacology 59: 1478-1485. [Crossref]
- Polly P, Herdick M, Moehren U, Baniahmad A, Heinzel T, et al (2000) Vdr-alien: a novel, dna-selective vitamin d-3 receptor-corepressor partnership. Faseb Journal 14: 1455-1463. [Crossref]
- Vaisanen S, Perakyla M, Kärkkäinen JI, Steinmeyer A, Carlberg C (2002) Critical role of helix 12 of the vitamin d-3 receptor for the partial agonism of carboxylic ester antagonists. Journal of Molecular Biology 315: 229-238. [Crossref]
- Zugel U, Steinmeyer A, Giesen C, Asadullah K (2002) A novel immunosuppressive 1 alpha,25-dihydroxyvitamin d-3 analog with reduced hypercalcemic activity. Journal of Investigative Dermatology 119: 1434-1442. [Crossref]
- Strauch UG, Obermeier F, Grunwald N, Dunger N, Rath HC, et al (2007) Calcitriol analog zk191784 ameliorates acute and chronic dextran sodium sulfate-induced colitis by modulation of intestinal dendritic cell numbers and phenotype. World Journal of Gastroenterology 13: 6529-6537. [Crossref]
- Martinesi M, Ambrosini S, Treves C, Zuegel U, Steinmeyer A, et al (2014) Role of vitamin d derivatives in intestinal tissue of patients with inflammatory bowel diseases. Journal Of Crohns & Colitis 8: 1062-1071. [Crossref]
- Herdick M, Steinmeyer A, Carlberg C (2000) Carboxylic ester antagonists of 1 alpha, 25-dihydroxyvitamin d-3 show cell-specific actions. Chemistry & Biology 7: 885-894. [Crossref]
- Schmitz AA, Hackethal S, Schulz A, May E, Steinmeyer A, et al (2015) Sharing pharma compounds with academia: experiences with providing vitamin d receptor ligands. Nature Reviews Drug Discovery 14: 294. [Crossref]
- Gustafson B, Hammarstedt A, Andersson CX, Smith U (2007) Inflamed adipose tissue - a culprit underlying the metabolic syndrome and atherosclerosis. Arteriosclerosis Thrombosis and Vascular Biology 27: 2276-2283. [Crossref]
- Gregor MF, Hotamisligil GS (2011) Inflammatory mechanisms in obesity. Annu Rev Immunol 29: 415-445. [Crossref]
- Fain JN (2006) Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam Horm 74: 443-477. [Crossref]
- Hotamisligil GS (2006) Inflammation and metabolic disorders. Nature 444: 860-867. [Crossref]
- Lira FS, Rosa JC, Cunha CA, Ribeiro EB, do Nascimento CO, et al (2011) Supplementing alpha-tocopherol (vitamin e) and vitamin D3 in high fat diet decrease il-6 production in murine epididymal adipose tissue and 3t3-l1 adipocytes following lps stimulation. Lipids Health Dis 10: 37. [Crossref]
- Mutt SJ, Karhu T, Lehtonen S, Lehenkari P, Carlberg C, et al (2012) Inhibition of cytokine secretion from adipocytes by 1,25-dihydroxyvitamin d(3) via the nf-kappab pathway. Faseb J 26: 4400-4407. [Crossref]
- Huckins D, Felson DT, Holick M (1990) Treatment of psoriatic-arthritis with oral 1,25-dihydroxyvitamin-D3 - a pilot-study. Arthritis and Rheumatism 33: 1723-1727. [Crossref]
- Chiang SH, Bazuine M, Lumeng CN, Geletka LM, Mowers J, et al (2009) The protein kinase ikk epsilon regulates energy balance in obese mice Cell 138: 961-975. [Crossref]
- Ding C, Gao D, Wilding J, Trayhurn P, Bing C (2012) Vitamin d signalling in adipose tissue. British Journal of Nutrition 108: 1915-1923. [Crossref]
- Tak PP, Firestein GS (2001) Nf-kappab: A key role in inflammatory diseases. J Clin Invest 107: 7-11. [Crossref]
- Herdick M, Steinmeyer A, Carlberg C (2000) Antagonistic action of a 25-carboxylic ester analogue of 1 alpha, 25-dihydroxyvitamin d-3 is mediated by a lack of ligand-induced vitamin d receptor interaction with coactivators. Journal of Biological Chemistry 275: 16506-16512. [Crossref]
- Molinari C, Rizzi M, Squarzanti DF, Pittarella P, Vacca G, et al (2013) 1 alpha, 25-dihydroxycholecalciferol (vitamin d3) induces no-dependent endothelial cell proliferation and migration in a three-dimensional matrix. Cellular Physiology and Biochemistry 31: 815-822. [Crossref]
- Sun L, Carpenter G (1998) Epidermal growth factor activation of nf-kappa b is mediated through i kappa b alpha degradation and intracellular free calcium. Oncogene 16: 2095-2102. [Crossref]
- Thuringer D, Hammann A, Benikhlef N, Fourmaux E, Bouchot A, et al (2011) Transactivation of the epidermal growth factor receptor by heat shock protein 90 via toll-like receptor 4 contributes to the migration of glioblastoma cells. Journal of Biological Chemistry 286: 3418-3428. [Crossref]
- Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, et al (2012) Vitamin d inhibits monocyte/macrophage proinflammatory cytokine production by targeting mapk phosphatase-1. J Immunol 188: 2127-2135. [Crossref]
- Gao D, Trayhurn P, Bing C (2010) Macrophage-secreted factors inhibit zag expression and secretion by human adipocytes. Mol Cell Endocrinol 325: 135-142. [Crossref]
- Rotter V, Nagaev I, Smith U (2003) Interleukin-6 (il-6) induces insulin resistance in 3t3-l1 adipocytes and is, like il-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem 278: 45777-45784. [Crossref]
- Kristiansen OP, Mandrup-poulsen T (2005) Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes 54 suppl 2: s114-124. [Crossref]
- Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, et al (2006) Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem 281: 26602-26614. [Crossref]
- Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, et al (2006) Mcp-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 116: 1494-1505. [Crossref]
- Waugh DJ, Wilson C (2008) The interleukin-8 pathway in cancer. Clin Cancer Res 14: 6735-6741. [Crossref]
- Neels JG, Badeanlou I, Hester KD, Samad F (2009) Keratinocyte-derived chemokine in obesity: expression, regulation, and role in adipose macrophage infiltration and glucose homeostasis. J Biol Chem 284: 20692-20698. [Crossref]
- Keophiphath M, Rouault C, Divoux A, Clément K, Lacasa D (2010) CCl5 promotes macrophage recruitment and survival in human adipose tissue. Arterioscler Thromb Vasc Biol 30: 39-45. [Crossref]







