Abstract
Objective: To evaluate in non-immunocompromised adults not vaccinated against HBV (Hepatitis B virus), the response rate to vaccination according to standard protocol (SP) (0, 1, 6 months) and response rate in non-responders subjected to two protocols.
Materials and methods: 192 employees in a Hospital located in Beirut, average age of 44.3, not previously vaccinated, non-immunocompromised, are vaccinated by Engerix B 20 mcg/1ml according to the SP. Non-responders to this protocol are divided into two subgroups. The first one receives a single booster dose 4 months after the end of the SP, and those who remains unresponsive receives a double booster dose 2 years later. The second one receives only one double booster dose 2 years after the SP.
Results: The rate of positive immune response after vaccination using the SP is 145/192 (75.5%). 28 non-responders’ individuals receives 4 months later a booster dose; the positive response rate is 9/28 (32.14%). The 19 non-responders individuals R1 receives a double booster at 2 years with a positive response rate of 7/18 (36.84%). The positive response in this subgroup is 57.14%. Non-responders formed by 19 individuals, are boosted after 2 years. Their positive response rate is 63.16%. 19/192 (9.9%) of the individuals who do not respond to any protocols. Female gender and advanced age are two factors that lower the response to vaccination.
Conclusion: A booster dose at 2 years gives a better immunity than a simple reminder at 4 months, and is similar to the protocol using a booster at 4 months followed by a second one after 2 years.
Key words
Hepatitis B, vaccinations, non-responders healthy adults, standard protocol, recall protocols, double dose
Introduction
Hepatitis B is a major contributor to the burden of infectious disease worldwide. An estimated 2 billion people are affected by hepatitis B virus (HBV) worldwide, according to the WHO [1]. During the acute phase, the infection varies from asymptomatic hepatitis to icteric hepatitis and sometimes fulminant. In the chronic phase, the spectrum of disease can range from healthy portage to chronic active hepatitis and its complications. HBV is the cause for nearly half of all cirrhosis diagnosis, 80% of hepatocellular carcinomas and 1 million deaths yearly worldwide [2]. HBV is up to a 100 times more transmissible than human immunodeficiency virus (HIV) [1]. It is transmitted through blood and bodily fluids. Infection occurs via three major routes: percutaneous or permucosal, sexual and vertical (from mother to child) [3]. Vaccination remains the most effective means for both prevention of infection and its sequelae.
Vaccines type
To date, three types of HBV vaccines are available. Krugman study on the immunogenicity of Hepatitis B surface antigen (HBsAg) and the protective effects of anti-HBs antibody against HBV eventually led to the generation of the first generation of vaccine containing inactivated derivative of HBsAg from the plasma of the humans infected with HBV. The vaccine was FDA approved in 1981 [4]. The recombinant DNA technology gave way to the second generation of HBV vaccines using the yeast Saccharomyces cerevisiae with these two formulas; Engerix B and HB Recombivax. These types of vaccinations also contain HBs Ag. However, third-generation vaccines are much more immunogenic of HBs Ag due to their use of pre-S1 and pre-S2 antigens, but they are still not widely used. They are also produced with recombinant desoxyribonucleic acid (DNA) technology in mammalian cells [4,5].
It is recognized that antibody levels of anti-HBs (Hepatitis B surface antibody) above 10 IU / l offer effective protection against the HBV [6]. The positive response rate after vaccination varies between 85 and 100% [7]. Several factors may reduce seroconversion: age, sex, weight, heredity, smoking, immunosuppression and the subcutaneous administration [4,5,8,9].
Injection site
The preferred injection site is the deltoid region in adults and thigh in children. The administration in the gluteal region should be avoided because it is associated with a decreased level of seroconversion [5].
Population at risk
Immunization, as a method of prevention, has been a major breakthrough in the global effort to eradicate the virus.
We must therefore offer vaccination to persons who, in the context of professional activities, are likely to be in directly or indirectly contact with patients exposed to blood and other biological products [10].
The policy of vaccination against HBV is based on two strategies:
-Identification and vaccination of persons at high risk of exposure;
-And, in view of longer-term control of hepatitis B, vaccination of infants and catch up vaccination for children and adolescents until the age of 15 who are not responsive to SP [10].
Possible target groups for catch-up vaccination could include certain birth cohorts and those exposed to risk factors [11].
Vaccination protocols
There are several vaccination protocols. The most adopted are: the standard protocol of 3 intramuscular injections at 0,1, and 6 months or the accelerated protocol with 3 IM (intra muscular) injections at 0,1, and 2 months with a booster dose at 1 year or the fast protocol by administrating 3 doses in 21 days (D0 (D: day), D7, D21 or D0, D10, D21 according to the marketing authorizations for the vaccines concerned), followed by a booster after 12 months, for the long term effect [4,10,12]. The recommended vaccine dosage is 20 mcg/ml.
In most cases, anti-HBs antibody levels which are between 100 and 1000 IU/l are achieved after a course of therapy, and some authorities recommend a fourth dose if the rates are between 10 and 100 IU / l. People who do not respond to the first series may benefit from additional doses. About 15 to 25% of people respond to an additional dose, and 30 to 50% to two additional doses [7].
Causes of non-responding to HBV vaccination
There may be a genetic predisposition for non-response. The human leukocyte antigen (HLA) along with MHC-II plays an important role in presentation of the viral peptides to CD-4 T-helper cells and subjects who fail to respond may have a defect in the antigen presentation or the stimulation of T-helper cells. Studies have shown that patients who are homozygous for HLA DRB1*0301, HLA-B8, SC01, DR-3, HLAB44, FC-31, DR-7 have an increased predisposition to non-responsiveness. Patients with advanced age, chronic diseases, immune defects or on immunomodulatory medications have a blunted immune response [1].
Objectives of this study
The objectives of this study are to assess, among not vaccinated immunocompetent adults:
1-The response rate to vaccination against HBV using the standard protocol.
2-The difference in response rate among people subjected to two protocols using a double dosage protocol.
3-The influence of the characteristics (age and sex) of the population in response to vaccination against HBV.
Materials and methods
The study is conducted in a hospital in Beirut on 192 employees with a median age of 44.3 (44.3 ± 12.35), not previously vaccinated.
The characteristics of the population
All individuals are between the age of 18 and 80 naïve of HBV vaccination with HBs antigen negative and negative HCV (Hepatitis C virus) serology with a median age of 44.3 years old. The population included 101 women and 91 men. All individuals presenting with anti-HBs antibody or anti HCV postitive are excluded as well as the patients on immunosuppressive treatment and or having a chronic disease such as cardiac failure, hepatic failure, chronic renal failure, HIV (Human immunodeficiency virus) infection, cancer or HCV infection.
Ethic committee
The protocol was submitted and accepted by the ethics committee of Saint-Joseph univerity, Beirut, Lebanon. All the patients received a lecture about the benefits of the HBV vaccine. Written consent was required to the patient before taking part in the study.
Blood tests
Laboratory tests with complete blood cells, urea, creatinine, SGPT, SGOT, GGT, alkaline phosphatase were performed by a registered nurse and were within normal range in the pre inclusion phase.
Serological assessment including HBsAg, anti-HBs antibody, anti-HBc antibody, HBe antigen, anti-HBe antibody, anti HCV and HIV status, returned negative.
Vaccination
The vaccine used and available on the Lebanese market is Engerix B (Engerix® B 20 mcg/1ml) containing the surface antigen produced in yeast cells (Saccharomyces cerevisiae) by recombinant DNA adsorbed on the hydrated aluminum hydroxide.
Vaccination is done intramuscularly in the deltoid region by a registered nurse.
Follow-up blood tests
Anti-HBs antibodies were measured 1 month after the end of each protocol of immunization and/or reminder. In our study, the rate of anti-HBs 10 IU/l was chosen as threshold protection against HBV infection. The individuals who has an anti-HBs level less than 10IU/l were considered not responders and without any immunity against the HBV.
Vaccination protocol
In our study, the immunization protocol adopted is day 0, after 1 month and after 6 months.
Non-responders to this protocol were divided into two subgroups by random draw:
The first subgroup (P1) received a single booster dose (R1) four months after the standard protocol, and those who remained unresponsive, a double booster dose of 40 micrograms (R1b), 2 years after the end of the standard protocol.
The second subgroup (P2) received only a double booster dose of 40 micrograms (R2) 2 years after the standard protocol (Figure 1).
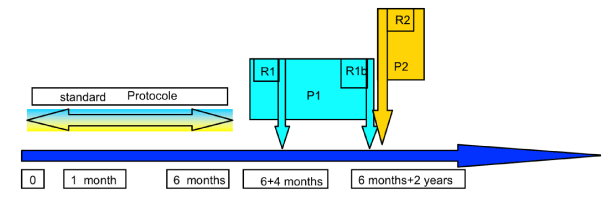
Figure 1. Summary of vaccine protocols
Data analysis
The final collection of information about all individuals is done on Excel software version 14.6.0. The processing of the results was performed using the SPSS program version 20 with Chi - 2 test, Student, exact Fisher, multiple logistic regression test, bivariate and multivariate analyzes to:
• Describe the characteristics (age and sex) of the population, and the respective answers after each step of immunization, according to the standard protocol and the various recall protocols.
• Compare the different recall protocols, and find if the age and sex significantly affect the response to vaccination.
Results
Rate of positive response according to SP
The rate of positive response after vaccination using the standard protocol is 75.5% (145/192) of which 83.3% (74/91) of men and 70.3% (71/101) of women.
Different branches of oghur study
The 47 non-responders to standard protocol individuals were randomly divided into two groups P1 and P2:
- 28 non-responders individuals (P1) were boosted R1 at four months, their positive response rate is 32.14% (9/28) of which 35% (5/14) are men and 28.6% (4/14) are women.
The 19 non-responders individuals R1 then received a reminder boosted (R1b) at 2 years. Their positive response rate was 36.84% (7/19), including 5 men and 2 women. The final rate of positive response (P1) is 57.14% (16/28) of which 10 men (71.4%) and 6 women (42.9%).
- 19 non-responders individuals (P2), were boosted R2 at 2 years. The positive response rate was 63.16% (12/19) of which 33.3% (1/3) were men and 68.9% (11/16) women (Figure 2).
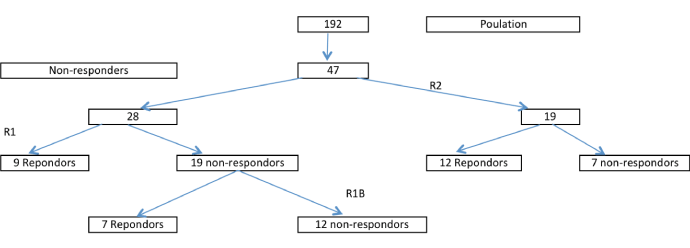
Figure 2. Population distribution in the different branches of study.
Comparison between the 2 protocols adapted in our study
The comparison of various reminders shows a significant difference between the responses R1 and R2 with a p value of 0.036 and no significant difference between the groups P1 (R1 + R1b) and P2 (R2) with a p-value of 0.767 (Figure 3).
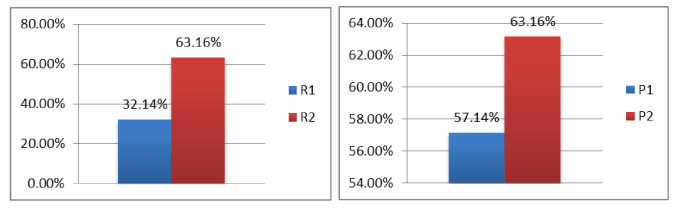
Figure 3. response comparison between the two protocols.
9.9% (19/192) of individuals have not responded to any of our recall protocols.
Comparison based on age and sex
Age and gender are significant determinants of response to vaccination with a p value = 0.0001 (significant difference).
Comparison between the sexes
There is no significant difference between the two sexes based on the standard protocol vaccination OR=1.83 (0.93-3.63).
By controlling the age factor, men have twice as positive response to vaccination than women after standard protocol. OR = 2.18 (1.06, 4.50). After each boosted dose, there is no significant difference in the response between men and women OR=2.52 (0.87-7.27)
Comparison between the age
The response decreases with age. This decrease was 5% each year with an OR = 0.95 (0.92, 0.97) and 41% every 10 years. OR = 0.59 (0.44, 0.78).
Based on sex: after every 1 year, the chance of response to vaccination decreases by 5% (according to the standard protocol). OR = 0.95 (0.92, 0 .97). The response decreases by 41% every 10 years with OR = 0.59 (0.44-0.78)
By controlling the sex factor, after every one year, the chance for a positive response falls 44%. OR = 0.56 (0.42, 0.75) (Tables 1 and 2).
| |
Variants |
OR (CI) |
p value |
Response to standard protocol |
Age |
0.94 (0.92-0.97) |
0 |
Sex |
1.83 (0.93-3.6) |
0.07 |
Response after standard protocol with boosting dose |
Age |
0.94 (0.91-0.98) |
0.007 |
Sex |
2.09 (0.76-5.75) |
0.153 |
Table 1. Bivariate analysis of the effect of gender and age on the response to vaccination using the standard protocol and the various recall protocols.
| |
Variants |
OR (CI) |
p value |
Response to standard protocol |
Age |
0.94 (0.91-0.97) |
0 |
2021 Copyright OAT. All rights reserv
Sex |
2.18 (1.05-4.5) |
0.034 |
Response after the standard protocol with boosting dose |
Age |
0.94 (0.90-0.98) |
0.004 |
Sex |
2.52 (0.87-7.27) |
0.086 |
Table 2. Multivariate analysis of the effect of gender and age on the response to vaccination using the standard protocol and the various recall protocols.
Discussion
Response to vaccination using the standard protocol
Several randomized double-blind studies have shown a seroconversion rate of 95% in healthy adults vaccinated according to the standard protocol [5,13]. In our series, the rate is 75.5%. The cause of this difference may be due to the fact that we have not studied the response by age. In fact, the response decreases with age, and the age of our population is between 22 and 76 years, which mean that the low response in the higher age groups will reduce the overall response to vaccination.
Response to vaccination after the recall protocol
The individuals who do not respond to standard immunization scheme are not protected against infection. Revaccination is recommended. Revaccination with a single booster dose in non-responders to standard protocol, gives a response rate of 15 to 25%. Two additional single doses (a total of 3 single doses) increase this rate up to 30 to 50% [7]. Comparing these results with those in our study, we find a rate of 32.14% after a single booster dose and 57.14% after three booster doses.
Although reminders up to 9 doses are recommended in non-responders healthy adults, it is not found in the literature a revaccination reference protocol [7].
By comparing the 2 reminder protocols P1 and P2 proposed in our study, it was found that in non-responders to standard protocol individuals, the fact of making a double dose recall at 2 years gives a better answer than that obtained after a reminder single dose at four months (63.16% v/s 32.14%) with a p-value = 0.036.
Moreover, if we consider the double dose recall at 2 years in non-responders individuals compared to the single dose reminder at four months (a total of three doses), the response rate increases by 32.14% to 57.14%, but not exceeding the obtained response with a single boosted recall (double dose) immediately at 2 years. (57.14% v / s 63.16%) with a p-value = 0.767.
One can therefore conclude that the factor that influences the response rate is the time interval to the recall booster rather than the dosage.
On the other hand, the recall at 4 months is still useful in providing immunity to 32.14% of non-responders for 2 years, which is the time before a reminder booster dose. This will provide them immunity to HBV.
9.9% (19 individuals) remains not immune to the HBV with the SP and the recall booster. More doses are recommended. More studies are needed to evaluate other recall protocols for this group.
Influence of age and sex to the vaccination response
There are several factors that can reduce the response to vaccination in healthy adults. These factors are: age over 40 years, smoking, obesity, male gender, and immunodeficiency. In addition, genetic factors are also involved [4,14,15].
In our series as in the global data, age and sex are both significant determinants of the response to the SP. P-value = 0.0001.
For the age, we notice a decreased response to vaccination as of 5% each year and 41% every 10 years. By adjusting the gender factor, this decrease will be 6% annually and 44% every 10 years.
By adjusting the sex factor, our results are in controversy with the literature since it was found that for the same age, men respond twice more than women after vaccination using the SP.
By making different booster protocols, we do not find a difference in response between the two sexes. This change of effect could be due to the fact that during the recall protocol P2, women respond better than men (68.9% vs 33.3 %) but the comparison between these two groups will be of low power because of the small number of individuals (one man v/s 11 women).
Limitation of the study
The number of our population is small and this study is conducted in one medical centre. We could perform a regional study that includes these parameters.
The influence of obesity and smoking were not studied in our work. We did not compare a double booster dose at 4 months in contrast with a single boost. The other limitation to our study is that the period between the prime and boost vaccination is too long to make patients immune against the HBV. The outcome for such individuals is to be careful while manipulating the blood.
Conclusion
The standard protocol for HBV vaccination is 0, 1 or 2, and 6 months. 5% of vaccinated patients are not responders. There is no standardized protocol for non-responders. A boosted reminder at 2 years gives a better answer than a simple reminder at four months, and a better response to a simple reminder at 4 months followed by a second booster at 2 years. However the booster dose at 4 months is important because it gives immunity to 32% of the non-responders. Female gender and advanced age are two factors that reduce the response to vaccination. 9.9% (19 individuals) in our study population remained unresponsive despite various reminders. Additional doses are recommended. Further studies would be interesting to follow up.
Funding
This article received no funding. The vaccines and the blood tests were offered by the hospital.
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
The protocol was submitted and accepted by the ethics committee of Saint-Joseph Univerity, Beirut, Lebanon.
Informed consent
Written informed consents were collected from all subjects before taking part of the protocols.
References
- Walayat S, Ahmed Z, Martin D, Puli S, Cashman M, et al. (2015) Recent advances in vaccination of non-responders to standard dose hepatitis B virus vaccine. World J Hepatol 7: 2503-2509. [Crossref]
- Lavanchy D (2004) Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat 11: 97-107. [Crossref]
- David MC, Ha SH, Paynter S, Lau C (2015) A systematic review and meta-analysis of management options for adults who respond poorly to hepatitis B vaccination. Vaccine 33: 6564-6569. [Crossref]
- Masters BR (2016). Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases, Eighth Edition (2015) Eds: John E. Bennett, Raphael Dolin, Martin J. Blaser. ISBN: 13-978-1-4557-4801-3, Elsevier Saunders. Graefes Arch Clin Exp Ophthalmol 254: 2285-2287. [Crossref]
- Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC (1999) What level of hepatitis B antibody is protective? J Infect Dis 179: 489-492. [Crossref]
- Sjogren MH (2005) Prevention of hepatitis B in nonresponders to initial hepatitis B virus vaccination. Am J Med 118 Suppl 10A: 34S-39S. [Crossref]
- Rezaee-Zavareh MS, Einollahi B (2014) Hepatitis B vaccination: needs a revision. Hepat Mon 14: e17461. [Crossref]
- DaRoza G, Loewen A, Djurdjev O, Love J, Kempston C, et al. (2003) Stage of chronic kidney disease predicts seroconversion after hepatitis B immunization: earlier is better. Am J Kidney Dis 42: 1184-1192. [Crossref]
- Domingo IG, Acevedo JMP, Vargas AA, Cuadra AR, Ríos MTF, et al. (2012) Response to vaccination against hepatitis B virus with a schedule of four 40-µg doses in cirrhotic patients evaluated for liver transplantation: factors associated with a response. Transplant Proc 44: 1499-1501. [Crossref]
- Calendrier vaccinal français.
- Kane MA (1998) Global status of HB immunization, 1998. Acta Gastroenterol Belg 61: 237. [Crossref]
- Jilg W, Schmidt M, Deinhardt F (1989) Vaccination against hepatitis B: comparison of three different vaccination schedules. J Infect Dis 160: 766-769. [Crossref]
- Yu AS, Cheung RC, Keeffe EB (2006) Hepatitis B vaccines. Infect Dis Clin North Am 20: 27-45. [Crossref]
- Wood RC, MacDonald KL, White KE, Hedberg CW, Hanson M, et al. (1993) Risk factors for lack of detectable antibody following hepatitis B vaccination of Minnesota health care workers. JAMA 270: 2935-2939. [Crossref]
- Höhler T, Reuss E, Evers N, Dietrich E, Rittner C, et al. (2002) Differential genetic determination of immune responsiveness to hepatitis B surface antigen and to hepatitis A virus: a vaccination study in twins. Lancet 360: 991-995. [Crossref]



