Abstract
Nesfastin-1 is a neuropeptide that may be involved in cardiovascular regulation. There are scarce data regarding serum nesfatin-1 concentrations and their relationship with cardiovascular disease in adolescents. The aim of this study was to investigate the association of nesfatin-1, body mass index and risk factors of cardiovascular disease in Mexican adolescents.
This is a cross-sectional study in adolescents between 15-19 years old, classified into 3 groups according to BMI and HOMA-IR: group 1 included 30 normal weight adolescents, group 2 (n=30) adolescents classified as metabolically healthy obese (MHO) and group 3 (n=42) metabolically unhealthy obese (MUO). Somatometric variables were evaluated and glucose, lipid profile, creatinine, insulin and nesfatin-1 were measured and HOMA-IR atherogenic index were calculated.
The results showed significant differences between the groups in the lipid profile p<0.001, triglycerides/ HDL-C and cholesterol / HDL-C indexes (p<0.0001) and nesfatin-1 (p<0.0007). A positive correlation was found between nesfatin-1, BMI (p<0.004), HOMA-IR (p<0.027), TG / HDL-C (p<0.012) and TC / HDL-C index (p<0.005).
In conclusion, adolescents with obesity have 3-fold higher levels of nesfatin-1 irrespective of insulin status. This peptide correlates with the classic cardiovascular risk factors such as BMI, HOMA-IR and the TG/HDL-C and TC/HDL-C index, which could pave the way for future studies exploring the potential of nesfatin-1 as an emerging cardiovascular risk marker.
Key words
atherogenic index, insulin resistance, metabolically healthy obese, nesfatin-1
Introduction
Obesity is a health problem that has increased in recent years to reach epidemic levels [1,2]. There is a subgroup within the population with obesity called metabolically healthy obese (MHO), which have an abnormal accumulation of subcutaneous fat, but no adverse metabolic effects, such as insulin resistance, dyslipidemia or hypertension [3,4]. With the increase in childhood obesity, the prevalence of other diseases such as type 2 diabetes mellitus, hypertension, non-alcoholic fatty liver and atherosclerotic cardiovascular diseases (ACVD) has increased [4]. Although ACVD and their clinical manifestations typically occur in adulthood, their risk factors are largely determined by behaviors learned in childhood and that persist through adulthood [5].
Emerging cardiovascular risk factors have been described. One of them is nesfatin-1, an 82 amino acid peptide. It is secreted in the hypothalamic nuclei associated with the melanocortin signaling pathway, mainly 3/4 receptors (MC3 / 4R). Nesfatin-1 is also secreted by peripheral tissues such as adipose tissue [6]. There are conflicting data regarding the secretion of nesfatin-1 in children and adolescents with obesity in the literature [7]. Dokumacioglu, et al. have shown that the concentration of nesfatin-1 is lower in obesity subjects than in control subjects [8]. Conversely, Anwar, et al. showed high concentration of nesfatin-1 in the group with obesity compare with the control group [7]. There are little data regarding nesfatin-1 secretion in obese young adolescents, so that it is of the utmost importance to know its status in this population, to ascertain whether Nesfatin-1 could be a new marker in the diagnosis and treatment of metabolic syndrome, obesity, diabetes and cardiovascular diseases [9]. Therefore, the aim of this study was to investigate the association of nesfatin-1, body mass index and risk factors of cardiovascular disease in Mexican adolescents with normal weight and obesity: metabolically healthy and unhealthy.
Material and methods
Participants
This comparative cross-sectional study was conducted between August 2018 and May 2019 in different educational institutions. It included 102 Mexican adolescents; between 15 to 19 years old, without chronic diseases, altered lipid profile, autoimmune, hormonal or infectious diseases. The participants were divided in 3 groups; group 1 included 30 normal weight adolescents, group 2 (n=30) adolescents classified as metabolically healthy obese (MHO) and group 3 (n=42) metabolically unhealthy obese (MUO) based on whether they had insulin resistance (IR) or not respectively [10]. Weight and height were measured following standardized methods, BMI was calculated as kg / m2, and the classification was made based on the WHO child growth charts. This study was approved for the Institutional Committee of Bioethics of the University of Guanajuato (CIBIUG-P24-2018). Both adolescents and their parents or tutors signed an informed consent form.
Biochemical measurements
A venous blood sample was obtained after 8-12 h of fasting and serum was processed the same day to measure glucose, (GOD-PAPTM, Lakeside, Mexico City) lipids by enzymatic methods (Spinreact, Spain), and the Schwartz-Lyon equation was used to calculate the estimation of glomerular filtration (eGF) [11].
Serum aliquots were stored at –80° C until further analyses. Insulin was measured by ELISA (ALPCOTM, Salem, NH, USA), and homeostatic model assessment-
insulin (HOMA-IR) was defined as above 95% percentile, according to a previous report in Mexican adolescents [12]. Nesfatin-1 levels were determined using a commercial ELISA kit (Phoenix Pharmaceuticals, Burlingame, Calif., USA).
To determine the alterations in the lipid profile was used the recommendations for children and adolescents of the National Cholesterol Education Program, total cholesterol <170 mg / dl, triglycerides <85 mg / dl, HDL-C > 35 mg / dl and glucose <90-130 mg / dl [13]. The triglycerides/HDL-C and total cholesterol/HDL-C indexes were calculated.
Statistical analysis
The distribution of the data was evaluated with the Shapiro-Wilk test and the results are expressed as median and interquartile range. Differences between groups were evaluated by the Kruskal Wallis test and the post hoc analysis by Tamhane test. Spearman correlation analysis was used to determine the univariate correlation between the different variables in the study. All analyses were performed using Statistica 7 software (StatSoft Inc., Tulsa, OK, USA). Significance was defined as a value of p < 0.05.
Results
A total of 1473 adolescents were screened for eligibility, of which 317 met the inclusion criteria; 72 adolescents with obesity and 64 with normal weight agreed to participate in the study. Within the group of adolescents with normal weight 34 werenot included in the study due to biochemical alterations (glucose, cholesterol, triglycerides, or HDL-C) and the final group included 30 teenagers with normal weight. The group of adolescents with obesity was divided into two groups as described in Methods: MHO (n=30) and MUO (n=42) respectively. By design, weight, BMI, lipid profile and HOMA-IR were significantly different between the groups of adolescents with obesity and normal weight; nesfatin-1 levels almost were 3-fold higher in obese adolescents as compared to lean counterparts (p<0.002) and no difference was found between metabolically healthy and unhealthy obese adolescents (Table 1). We evaluated the levels of nesfatin-1 and HOMA-IR between men and women without finding a significant difference between both groups.
Variable |
Normal weight
(n=30) |
MHO
(n=30) |
MUO
(n=42) |
P |
Age, (years) |
16 (2) |
16(2) |
16 (1) |
0.412 |
Female, n (%) |
13 (43.33) |
19 (63.33) |
27 (64.28) |
|
|
Median ( IQ range) |
Median ( IQ range) |
Median ( IQ range) |
|
Weight, (kg) |
55 (11.10)*¥ |
79.80 (14)*£ |
90.15 (15.80)¥£ |
<0.0001 |
Height, (m) |
1.65 (0.18) |
1.62 (0.09) |
1.63 (0.09) |
0.847 |
BMI, (kg/m2) |
20.62 (2.51)*¥ |
29.41 (2.06)*£ |
32.35 (4.96)¥£ |
<0.0001 |
Glucose, (mg/dl) |
92 (11) |
88 (13)£ |
96 (11)£ |
0.002 |
Total cholesterol, (mg/dl) |
149.50 (42)¥ |
156.50 (33)£ |
174 (39)¥£ |
0.0002 |
LDL-C, (mg/dl) |
87.50 (45)¥ |
93 (29)£ |
107 (37)¥£ |
0.001 |
HDL-C, (mg/dl) |
49 (14)¥ |
42 (8) |
41 (10)¥ |
0.0017 |
Triglycerides, (mg/dl) |
56 (16)*¥ |
80.50 (60)*£ |
120.50 (94)¥£ |
<0.0001 |
Insulin (μlU/mL) |
5.23 (4.38)*¥ |
9.15 (3.73)*£ |
24.47 (3.57)¥£ |
<0.0001 |
HOMA-IR |
1.17 (0.93)*¥ |
1.79 (1.12)*£ |
5.85 (3.57)¥£ |
<0.0001 |
Nesfatin-1 (pg/ml) |
18.08 (33.45)Φδ |
55.24 (67.06)Φ |
56.90 (66.70)δ |
0.0002 |
eGF Schwartz-Lyon (ml/min) |
80.30 (86.21) |
82.08 (88.50) |
83.08 (94.40) |
0.271 |
TG/HDL-C index |
1.19 (0.44)*¥ |
1.91 (1.52)*£ |
3.19 (2.36)¥£ |
<0.0001 |
TC/HDL-C index |
3.15 (0.92) *¥ |
3.82 (1.03)*£ |
4.34 (1.21)¥£ |
<0.0001 |
MHO, metabolically healthy obese adolescents; MUO, metabolically unhealthy obese adolescents; BMI, body mass index; LDL-C, low density lipoprotein; HDL-C, high density lipoprotein; TG, triglycerides; TC, total cholesterol; eGF estimation of glomerular filtration. *p <0.01 between normal weight group and MHO; ¥p <0.01 between normal weight group and MUO; £p <0.01 between MHO and MUO; Φp = 0.39 between normal weight group and MHO; δp = 0.034 between normal weight group and MUO
Table 1. Anthropometric and metabolic variables in the study group
Table 2 shows the results of the correlation between nesfatin-1 and the variables in the study in the whole group. We found a positive correlation between nesfatin-1 and BMI (Figure 1, panel A), as well as HOMA-IR, triglycerides, TG/HDL-C and TC/HDL-C index (Figure 1, panel B) a negative correlation with HDL-C.
Variable |
Rho |
P |
Nesfatin-1 |
|
|
BMI |
0.307 |
<0.001 |
HOMA-IR |
0.221 |
<0.025 |
Total cholesterol |
0.104 |
0.296 |
HDL-C |
-0.220 |
<0.026 |
LDL-C |
0.111 |
0.266 |
Triglycerides |
0.218 |
<0.027 |
TG/HDL-C index |
0.250 |
<0.011 |
TC/HDL-C index |
0.271 |
<0.005 |
Table 2. Correlation between nesfatin-1, BMI, HOMA-IR, lipid profile and atherogenic index in the whole group
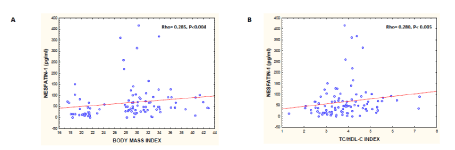
Figure 1. Correlation between nesfatin-1, BMI and triglycerides/HDL-C; Panel A shows Correlation between nesfatin-1 and body mass index; Panel B shows Correlation between nesfatin-1 and TC/HDL-C index
Discussion
To our knowledge, this is the first study that evaluates nesfatin-1 in MHO and MUO. It confirms previously reported higher values in obese vs lean adolescents and found no biologically significant difference when comparing MUO with MHO. We also found positive correlations with surrogate markers of cardiometabolic dyslipidemia in this young population.
The ability of BMI to predict future cardiovascular diseases in childhood is remarkably consistent, indeed, sometimes exceeding other risk factors for cardiovascular disease in adults [14-18]. Our study group with obesity was divided according to the presence or absence of insulin resistance in MUO and MHO, as well as alterations in the lipid profile and plasma glucose according to the Prince, et al. [19] and Ding, et al. [20]. We found a positive correlation between nesfatin-1 and HOMA-IR. We did not find significant differences between genders for HOMA-IR, contrary to Aradillas, et al. who in a study conducted in a population with Mexican children and adolescents, found a significantly higher average of HOMA-IR in the female gender compared to the male gender [12]. Ramanjaneya, et al. in an ex vivo model of subcutaneous adipose tissue, observed that the production of nesfatin-1 was significantly increased by insulin and dexamethasone, as well as by interleukin-6 (IL-6) and tumor necrosis factor alpha (TNFα). Insulin induces PPARy expression, similarly it has been shown that PPARy agonists induce NUCB2 activation [16]. PPARy also has a share in the increment of fat percentage through pubertal development [17]. Thus, the association between nesfatin-1 and this transcriptional factor may help explain the positive correlation found in our study for both BMI and HOMA-IR.
Our results show significantly higher levels of nesfatin-1 in adolescents with obesity between the ages of 15 and 19 compared to controls. These findings are in agreement with Anwar, et al. [7]. On the other hand, contrary to our results, Abaci, et al. [18] and Kim, et al. [19] shown lower concentrations of nesfatin-1 in obese subjects compared to controls. The age difference in the participants in various studies may partially explain why these results differ from previous research, since nesfatin-1 is not only secreted at the level of the central nervous system but in multiple peripheral organs such as adipose tissue, pancreas and testicles [6].
It has been reported that nesfatin-1 crosses the blood-brain barrier and that its reabsorption in cerebrospinal fluid is compromised in obese individuals due to saturation of the receptors, resulting in an even greater accumulation of this peptide in plasma [20-23]. This combination of factors could explain the higher levels of nesfatin-1 in adolescents with obesity shown in our study.
It has been described that the TG/HDL-C (surrogate marker of cardiometabolic dyslipidaemia: i.e., small-dense LDL) and TC/HDL-C indexes could be useful markers to identify atherogenic risk [24,25]. Yin, et al. shown that in addition to its involvement in insulin and glucose metabolism, nesfatin-1 plays a role in the regulation of peripheral lipid accumulation and hepatic lipid metabolism in mice [23] and Tekin, et al. associated nesfatin-1 with metabolic syndrome and its components [9]. In this sense, in the present study, higher levels of nesfatin-1 and higher values of cardiometabolic dyslipidaemia markers were found in MUO adolescents, suggesting that this group is more prone to developing CVD,
Due to the role of sex hormones such as LH and FSH during adolescence, we compared the concentrations of nesfatin-1 between males and females without finding a significant difference between both groups. There is a paucity of human studies that report whether nesfatin-1 is involved in pubertal development or not. Studies such as those by Ayca Altincik, et al. and G. Catli, et al. have failed to show any correlation between nesfatin-1 and FSH, LH and oestradiol in girls and adolescents [26].
The multicentre study of Pathobiological Determinants of Atherosclerosis in Youth (PDAY) refers to the relationship of risk factors in adolescents and young adults with atherosclerosis. In this cohort, non-HDL cholesterol was positively associated and HDL-C negatively, with lesions in the right coronary artery [26]. Our results showed significant differences between the three study groups with respect to triglycerides concentrations, while HDL-C only showed significant differences between the control group and the MUO group and show a positive correlation between nesfatin-1 vs triglycerides and the TG/HDL-C and TC/HDL-C index, which could suggest a role of nesfatin-1 as a cardiovascular risk factor. These results are in disagreement with what was reported in teenagers by Abaci, et al. [18] and by Kim, et al. [19] who did not show significant correlation between nesfatin-1, triglycerides. These differences in the results can be explained by age differences since they include children from 5 years old and our groups are adolescents.
One limitation of the study was that blood pressure and waist circumference were not measured.
Conclusion
In conclusion, this work shows that the concentrations of nesfatin-1 in adolescents with obesity are higher compared to adolescents with normal weight, but not between MHO and MUO adolescents. This peptide correlates with the classic cardiovascular risk factors such as BMI, HOMA-IR and the TG/HDL-C and TC/HDL-C index, which could pave the way for future studies exploring the potential of nesfatin-1 as an emerging cardiovascular risk marker.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
This work was supported by the University of Guanajuato (grant number DAIP-302/2018).
References
- www.who.int/es/news-room/fact-sheets/detail/obesity-and-overweight
- van Vliet-Ostaptchouk JV, Nuotio ML, Slagter SN, Doiron D, Fischer K, et al. (2014) The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: A collaborative analysis of ten large cohort studies. BMC Endocr Disord 14: 9. [Crossref]
- Stefan N, Häring HU, Hu FB, Schulze MB (2013) Metabolically healthy obesity: Epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol 1: 152-162. [Crossref]
- Kimm SYS, Barton BA, Obarzanek E, McMahon RP, Kronsberg SS, et al. (2002) Obesity development during adolescence in a biracial cohort: the NHLBI Growth and Health Study. Pediatrics 110: e54. [Crossref]
- http://www.who.int/cardiovascular_diseases/ resources /atlas/en/
- Stengel A, Taché Y (2010) Nesfatin-1 - Role as possible new potent regulator of food intake. Regul Pept 163: 18-23. [Crossref]
- Anwar GM, Yamamah G, Ibrahim A, El-Lebedy D, Farid TM, et al. (2014) Nesfatin-1 in childhood and adolescent obesity and its association with food intake, body composition and insulin resistance. Regul Pept 188: 21-24. [Crossref]
- Dokumacioglu E, Iskender H, Sahin A, Erturk EY, Kaynar O (2020) Serum levels of nesfatin-1 and irisin in obese children. Eur Cytokine Netw 31: 39-43. [Crossref]
- Tekin T, Cicek B, Konyaligil N (2019) Regulatory peptide Nesfatin-1 and its relationship with metabolic syndrome. Eurasian J Med 51: 280-284. [Crossref]
- Ding WQ, Yan YK, Zhang MX, Cheng H, Zhao XY, et al. (2015) Hypertension outcomes in metabolically unhealthy normal-weight and metabolically healthy obese children and adolescents. J Hum Hypertens 29: 548-554. [Crossref]
- de Souza VC, Rabilloud M, Cochat P, Selistre L, Hadj-Aissa A, et al. (2012) Schwartz Formula: Is One k-Coefficient Adequate for All Children? PLoS One 7: e53439. [Crossref]
- Aradillas-García C, Rodríguez-Morán M, Garay-Sevilla ME, Malacara JM, Rascon-Pacheco RA, et al. (2012) Distribution of the homeostasis model assessment of insulin resistance in Mexican children and adolescents. Eur J Endocrinol 166: 301-306. [Crossref]
- Gómez CZ, Romero VE, Hernández TA, Verdín SH, Figueroa GRM, et al. (2013) Estado nutricional y perfil de lípidos en adolescentes de un escuela rural. Rev Mex Pediatría 80: 5-9.
- Olson M, Chambers M, Shaibi G (2018) Pediatric Markers of Adult Cardiovascular Disease. Curr Pediatr Rev 13: 255-259. [Crossref]
- Prince RL, Kuk JL, Ambler KA, Dhaliwal J, Ball GDC (2014) Predictors of metabolically healthy obesity in children. Diabetes Care 37: 1462-1468. [Crossref]
- Ramanjaneya M, Chen J, Brown JE, Tripathi G, Hallschmid M, et al. (2010) Identification of nesfatin-1 in human and murine adipose tissue: A novel depot-specific adipokine with increased levels in obesity. Endocrinology 151: 3169-3180. [Crossref]
- Mirzaei K, Hossein-nezhad A, Keshavarz SA, Koohdani F, Eshraghian MR, et al. (2015) Association of nesfatin-1 level with body composition, dietary intake and resting metabolic rate in obese and morbid obese subjects. Diabetes Metab Syndr 9: 292-298. [Crossref]
- Abaci A, Catli G, Anik A, Kume T, Bober E (2013) The relation of serum nesfatin-1 level with metabolic and clinical parameters in obese and healthy children. Pediatr Diabetes 14: 189-195. [Crossref]
- Kim SH, Ahn MB, Cho WK, Cho KS, Jung MH, et al. (2019) The relation of serum nesfatin-1 level with anthropometric and metabolic parameters in children and adolescents: A prospective observational study. Medicine 98: e15460. [Crossref]
- Tan BK, Hallschmid M, Kern W, Lehnert H, Randeva HS (2011) Decreased cerebrospinal fluid/plasma ratio of the novel satiety molecule, nesfatin-1/NUCB-2, in obese humans: Evidence of nesfatin-1/NUCB-2 resistance and implications for obesity treatment. J Clin Endocrinol Metab 96: 669-673. [Crossref]
- Quijada Z, Paoli M, Zerpa Y, Camacho N, Cichetti R, et al. (2008) The triglyceride/HDL-cholesterol ratio as a marker of cardiovascular risk in obese children; association with traditional and emergent risk factors. Pediatr Diabetes 9: 464-471. [Crossref]
- Millan J, Pintó X, Muñoz A, Zúñiga M, Rubiés-Prat J, et al. (2009) Lipoprotein ratios: Physiological significance and clinical usefulness in cardiovascular prevention. Vasc Health Risk Manag 5: 757-765. [Crossref]
- Yin Y, Li Z, Gao L, Li Y, Zhao J, et al. (2015) AMPK-dependent modulation of hepatic lipid metabolism by nesfatin-1. Mol Cell Endocrinol 417: 20-26. [Crossref]
- Altıncık A, Sayin O (2018) Serum nesfatin-1 levels in girls with idiopathic central precocious puberty. J Clin Res Pediatr Endocrinol 10: 8-12. [Crossref]
- Çatll G, Anık A, Küme T, Çalan OG, Dündar BN, et al. (2015) Serum nesfatin-1 and leptin levels in non-obese girls with premature thelarche. J Endocrinol Invest 38: 909-913. [Crossref]
- Wissler RW (1991) USA Multicenter study of the pathobiology of atherosclerosis in youth. Ann N Y Acad Sci 623: 26-39. [Crossref]
