Introduction: Menstruation has long been an imperative societal indicator of female sexual development. Its absence is an important gynaecological problem requiring expert and adjuvant evaluation. Laparoscopy therefore offers a vital and invaluable means to the gynaecologist, in his quest for the accurate and genuine cause of primary amenorrhoea, in addition to clinical assessment and certain biochemical investigations.
Cases:We report a case series involving seven cases of primary amenorrhoea that presented in Life Institute For Endoscopy Limited, Nnewi (private specialist gynaecological endoscopy centre) and had laparoscopy evaluation between February 2006 and January 2011(a six –year period). They were between the ages of 16 and 30 years with a mean age of 24.86 years. Three were married and had fertility challenge while four were still single. Two were diagnosed with ovarian agenesis, two with ovarian dysgenesis, and one each with mullerian duct dysgenesis and agenesis while one patient had a combination of mullerian duct dysgenesis and ovarian agenesis.
Conclusion: Primary amenorrhoea remains a serious gynaecological concern. Laparoscopy evaluation as shown in these cases is an adequate and invaluable evaluation to unravel the anatomical cause of this anomaly and even propose possible line of treatment.
primary amenorrhoea, bucal smear, diagnostic laparoscopy
Menstruation has long been an important societal marker of female sexual development [1]. Amenorrhoea literally means absence of menses. It could be primary or secondary. Primary amenorrhoea is defined as the absence of menstruation by the age of 14 years in the absence of secondary sexual characteristics or the absence of menstruation by 16 years regardless of the presence or absence of secondary sexual characteristics [1-3]. In evaluating a patient for primary amenorrhoea, the differential diagnosis must include the anatomical developmental abnormalities. It is an important cause of primary infertility in females. We report a case series of primary amenorrhoea that presented in Life Institute For Endoscopy Limited, Nnewi, Nigeria (private specialist gynaecological endoscopy center) and had diagnostic laparoscopy with/ without dye tests.
Case 1
Miss UT was a 24- year old nullipara, a secondary school student who was referred to our center due to absence of menstruation as at her present age. She was considering starting a courtship with her intending husband, but was concerned about her fertility. She had no other medical or gynaecological problem. Miss UT had no history of previous surgery.
On examination she had poor development of secondary sexual characteristics. The breast was Tanner stage II, pubic hair was Tanner stage III, while there was no axillary hair growth. The abdomen was normal. Inspection of her genitalia revealed infantile external genitalia. Speculum examination revealed a normal vagina and a very tiny cervix. On bimanual palpation, the uterus was not palpable.
Biochemical investigations revealed normal thyroid function, prolactin levels, and elevated LH and FSH levels. The oestrogen and progesterone levels were low. Karyotyping was not done due to lack of facility in our center but buccal smear showed chromatin negative.
Transabdominal ultrasound was done and it revealed bilateral absent ovaries, with infantile uterus. There was no abnormality in the renal tract.
Laparoscopy and dye test revealed infantile cervix and uterus but well developed uterine appendages. There was no other uterine mass. The tubes were seen bilaterally but were tiny. There was absent ovaries. The dye test showed prompt and bilateral spillage of dyes. A diagnosis of congenital absence of the ovary was made.
The patient and parents were counseled on the diagnosis and they desired that the patient be managed so as to achieve secondary sexual characteristics. The problem of infertility was discussed and she consented to hormone therapy. She was lost to follow-up.
Case 2
Mrs NC was a 27 –year old nulliparous teacher, who presented with primary amenorrhoea and cyclical breast tenderness. She was considering starting a family with her husband but she was concerned about her fertility. She had no other medical or gynaecological problems. Her married elder sibling also had primary infertility and primary amenorrhoea as at the age of 33.
On examination she had well developed secondary sexual characteristics. The breast was Tanner stage IV, pubic hair was tanner stage IV, while there was normal axillary hair growth. There was no expressible galactorrhoea. The abdomen was normal. Inspection of her genitalia revealed normal female external genitalia. Speculum examination revealed a normal vagina but an infantile cervix. On bimanual palpation, the cervix and uterus were infantile.
Biochemical investigations revealed normal thyroid function, progesterone, and prolactin levels, but raised LH and FSH levels. The oestrogen level was low. Karyotyping was not done due to lack of facility in our center but buccal smear showed chromatin negative. As at presentation, the patient has been reared as female.
Transabdominal ultrasound revealed bilateral streak ovaries, and infantile uterus. There was no abnormality in the renal tract.
Laparoscopy and dye test revealed nulliparous cervical os, small but well developed uterus. There was no uterine mass. The tubes were seen bilaterally but were convoluted. The ovaries were streak bilaterally. The dye test showed prompt and bilateral spillage of dye. A diagnosis of primary amenorrhoea secondary to ovarian dysgenesis was made.
The patient and her husband were counseled on the diagnosis and the need for in vitro-fertilization and embryo transfer with ovum donation. The patient preferred the treatment for initiation of menstruation and she was placed on hormone therapy. She was lost to follow-up.
Case 3
Miss NC was a 26 year-old nullipara, an undergraduate who presented with primary amenorrhoea. She was considering starting a courtship with her intending husband, but was concerned about repeated failed penetration at attempted coitus. She had been evaluated prior to presentation in another hospital with ultrasound findings suggestive of ovarian cyst and ovo-testis. There was history of delayed menarche in the mother and the elder sister who is 28 years had primary amenorrhoea. She had no other medical or gynaecological problem.
On examination she had well developed secondary sexual characteristics. The breast and pubic hair were Tanner stage IV, while the axillary hair growth was Tanner stage III. The abdomen was normal. The clitoris, labia majora and minora and vestibule were normal. Speculum examination revealed a blind ending vagina and no cervix. On bimanual palpation, only the 3 cm depth of the vagina was felt, without any palpable uterus.
Biochemical investigations revealed normal progesterone, oestrogen and prolactin levels, and raised LH and FSH levels. Karyotyping was not done due to lack of facility in our center but buccal smear showed chromatin positive.
Transabdominal ultrasound revealed bilateral streak ovaries, and infantile uterus. There was no abnormality in the renal tract.
Laparoscopy revealed blind ending lower third of the vagina and absent uterus. There was no other pelvic mass. Rudimentary tubes were seen bilaterally. The ovaries were normal and well developed with corpus luteum bilaterally. The dye test was not done due to blind ending vagina. A diagnosis of primary amenorrhoea secondary to mullerian agenesis and normal functioning ovaries was made (Figure 1).
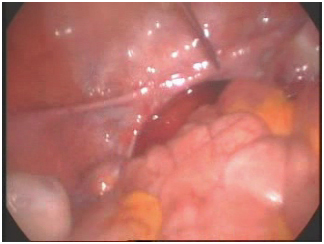
Figure 1. Absent uterus.
The patient and intending husband were counseled on the diagnosis and the need for gestational surrogacy using their ovum/ semen. She was counselled on serial vaginal dialatation in the meantime. However, she was lost to follow-up.
Case 4
Miss MN was a 30- year-old nullipara, a fashion designer who was referred to our facility due to primary amenorrhoea. She had been evaluated prior to presentation in the referral hospital with ultrasound findings suggestive of ovarian pathology. She had been on hormonal to induce menstruation before presentation but to no avail. She had no other medical or gynaecological problem.
On examination she was obesed and had well developed secondary sexual characteristics. The breast and pubic hair were Tanner stage IV, while the axillary hair growth was Tanner stage III. The abdomen was normal. The clitoris, labia majora and minora and vestibule were normal. Speculum examination revealed normal vagina but cervix was not seen. On bimanual palpation, the cervix and uterus were infantile and the adnexae were free.
Biochemical investigations revealed low progesterone, oestrogen and prolactin levels, normal LH level but raised FSH level. Karyotyping was not done due to lack of facility in our center but buccal smear showed chromatin negative.
Transabdominal ultrasound revealed absent ovaries, and rudimentary uterus. There was no abnormality in the renal tract.
Laparoscopy and dye test revealed very small cervix and rudimentary uterus, with cervical os to fundus diameter of 5.5 cm. The uterus tapered to a pointed fundus. The broad ligaments were normal. The tubes were absent except for the thickening on the superior part of the fold of the broad ligament stretching from the side walls to the uterus. The ovaries were absent. The cul-de-sac was clear. The dye test was not done. A diagnosis of primary amenorrhoea secondary to proximal paramesonephric duct and gonadal agenesis was made.
The patient and intending husband were counseled on the diagnosis and she desired to menstruate. The amenorrhoea persisted for up to 5 months on hormone therapy. However, she did not turn up for follow-up visit subsequently.
Case 5
Miss EI was a 23- year-old nulliparous undergraduate who presented with primary amenorrhoea. She had been evaluated prior to presentation in a teaching hospital with ultrasound findings suggestive of absent ovaries and small uterus. There was no family history of delayed menarche or primary amenorrhoea. She had no other medical or gynaecological problem.
On examination she had poorly developed secondary sexual characteristics. The breast and pubic hair were Tanner stage III, while the axillary hair growth was Tanner stage II. The abdomen was normal. The clitoris, labia majora and minora and vestibule were normal. Speculum examination revealed a small cervix. On bimanual palpation, it was not possible to palpate the uterus.
Biochemical investigations revealed low progesterone, oestrogen and prolactin levels, but raised LH and FSH levels. Buccal smear showed chromatin positive.
Pelvic ultrasound was done and it revealed bilateral streak ovaries, and infantile uterus. There was no abnormality in the renal tract.
Laparoscopy and dye test revealed an infantile uterus and no uterine mass. The tubes were seen bilaterally but were rudimentary and bilaterally patent on dye test. There was a streak ovary on the right and absent ovary on the left side. A diagnosis of primary amenorrhoea secondary to ovarian dysgenesis was made.
The patient and her mother were counseled on the diagnosis and the need for hormone replacement therapy. She is still on follow up.
Case 6
Miss UU was a 16- year-old senior secondary school student who presented with primary amenorrhoea. She was concerned about her inability to menstruate as at her present age. She had been evaluated prior to presentation in a teaching hospital with ultrasound findings suggestive of absent ovaries and small sized uterus. She had no family history of delayed menarche or primary amenorrhoea. She had no other medical or gynaecological problem.
On examination she had very poorly developed secondary sexual characteristics. The breast and pubic hair were Tanner stage II, while the axillary hair growth was absent (Tanner stage I). The abdomen was normal. The clitoris, labia majora and minora and vestibule were normal. Speculum examination revealed a well-developed vagina and infantile cervix. On bimanual palpation, the uterus was not palpable but the cervix was felt.
Biochemical investigations revealed low progesterone and oestrogen, normal prolactin levels, and raised LH and FSH levels. The testosterone level was normal. Buccal smear showed chromatin negative.
Pelvic ultrasound revealed bilateral absent ovaries, and infantile uterus that measured 33 X 23 cm. There was no abnormality in the renal tract.
Laparoscopy and dye test revealed normal vagina and infantile uterus. The tubes were seen bilaterally but appear rudimentary. The ovaries were absent. The dye test showed bilateral patent tubes. A diagnosis of primary amenorrhoea secondary to ovarian agenesis was made.
They were counseled on the diagnosis and the need for hormone replacement therapy. However, she was lost to follow-up.
Case 7
2021 Copyright OAT. All rights reserv
Mrs SU was a 28- year-old undergraduate who presented with primary amenorrhoea. She was already married but was concerned about her failed penetration at attempted coitus. There was no family history of delayed menarche or primary amenorrhoea. She had no other medical or gynaecological problem.
On examination she had well developed secondary sexual characteristics. The breast and pubic hair were Tanner stage IV, while the axillary hair growth was Tanner stage III. The abdomen was normal. The clitoris, labia majora and minora and vestibule were normal. Speculum examination revealed a blind ending vagina and cervix was not seen. On bimanual palpation, the uterus was not palpable.
Biochemical investigations revealed normal progesterone, oestrogen and prolactin levels, but raised LH and FSH levels. Karyotyping was not done due to lack of facility in our center but buccal smear showed chromatin positive.
Pelvic ultrasound was done and it revealed bilateral normal ovaries, and infantile uterus. There was no abnormality in the renal tract.
Laparoscopy revealed blind ending one-third of the lower vagina and double uterus. There was no other uterine mass. The tubes were seen bilaterally as normal except that the left tube had atrophic isthmic portion. The ovaries were normal and well developed. The dye test was not done due to blind ending vagina. A diagnosis of primary amenorrhoea secondary to mullerian dysgenesis and normal functioning ovaries was made (Figure 2).
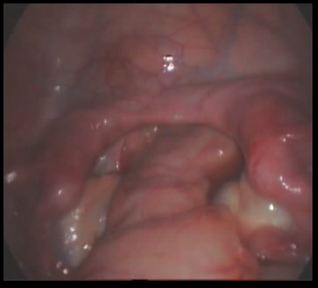
Figure 2. Mulerian dysgenesis.
The patient and her husband were counseled on the diagnosis and the benefit of In Vitro Fertilization (IVF) and serial vaginal dialatation. However, she was lost to follow-up.
Laparoscopy offers a vital and invaluable means to the gynaecologist, in his quest for the accurate cause of primary amenorrhoea, in addition to clinical assessment and certain biochemical investigations [2,4]. Clinical examination is often inadequate for a definitive diagnosis. High-tech investigations, such as chromosomal studies, ovarian stimulation tests with gonadotrophins, gonadotrophin assays, and leuteinising hormone releasing factor are not constantly at the reach of all evaluating centers, due largely to inadequate and non-availability of diagnostic facilities [4]. Diagnostic laparoscopy in conjunction with pelvic ultrasound, hormone assay and buccal smear were used judiciously in our resource constrained environment to determine the genuine cause of the amenorrhoea. Early evaluation is recommended for these women to allow for treatment before they advance in age and marry ignorantly.
Thus, primary amenorrhoea remains a well-recognized gynaecological entity that often constitutes a serious source of worry to the patients, intending husbands, parents and the gynaecologist. It is a common cause of primary infertility in females and does not have any racial predilection. Many patients with primary amenorrhoea do not receive the diagnosis until they are adults as in our patients.
A number of conditions could present with primary amenorrhoea in an adult. In situations where there are presence of normal secondary sexual characteristics, primary amenorrhoea could be due to outflow tract abnormalities (such as imperforate hymen, transverse vaginal septum) or XY female (androgen insensitivity), resistant ovary syndrome, and absent vagina with non-functioning uterus (Meyer-Rokitansky-Kuster-Hauser syndrome (cases 3 and 7)) [2]. Constitutional delay could also be a cause. However, in cases where the secondary sexual characteristics are absent, primary amenorrhoea could be due to gonadal agenesis (cases 4 and 6) or dysgenesis (case 5), turner syndrome (cases 1 and 2)/mosaic, ovarian failure, hyperprolactinaemia, and olfactogenital syndrome [1,3]. Of the above, Turner syndrome is the most common cause, followed by Mayer-Rokitansky-Kuster-Hauser syndrome [1,3].
In some cases, primary amenorrhoea may be associated with heterosexual characteristics. These include congenital adrenal hyperplasia, 5-alpha reductase inhibitor, and partial androgen receptor deficiency [3,5]. None of our patients had these latter characteristics as they were already adults at presentation and diagnosis. Low Tanner stages for both breast and pubic hair development indicates that all aspects of puberty are delayed, including menstrual function. If both Tanner stages are high, it implies that puberty has progressed normally with the exception of menstrual function. If the Tanner stages are divergent (for example, breast V and pubic hair I) there has been asynchrony in the oestrogen and androgen dependent aspect of puberty [1].
In evaluation and management of primary amenorrhoea, it is important to record a full history and examination including most importantly the development of secondary sexual characteristics. The presence of normal secondary sexual characteristics should alert the obstetrician of the likelihood of an outflow tract obstruction. This is the most common cause of primary amenorrhoea in the presence of secondary sexual characteristics [3] as we observed in almost all the patients in this case series.
Additionally, in this case series, the presence of the secondary sexual characteristics could not correlate with all the laparoscopic findings. This may be that before presentation some of the patients had been on hormone replacement therapy. However, the role of laparoscopy is invaluable [6,7] since none of the patients could be diagnosed until adulthood.
The complete forms of androgen insensitivity are associated with amenorrhoea and normal breast development. Affected persons have normal testicular function but are not responsive to male concentrations of testosterone, and the development of breasts is secondary to the small amounts of unopposed oestrogens produced by the testis. The pubic and axillary hair is scanty or often absent because the pubic hair and axillary hair growth appear to be under the control of adrenal androgens [4,8]. A short blind vaginal pouch is usually present [8].
In Turner's syndrome, the oestrogen and androgen levels are decreased and FSH and LH levels are increased [7]. However, the oestrogen-dependent organs show the predictable effects of hormonal deficiency. The breasts will contain little parenchymal tissue, and the areolar tissue is only slightly darker than the surrounding skin. The external genitalia, vagina, and müllerian derivatives will be well-differentiated but will remain small. The pubic and axillary hairs usually fail to develop in normal quantity [4,8].
However, normal pubertal development, menstruation, and even pregnancies have been reported in adults with gonadal dysgenesis [7]. It is possible that a few of these persons maintain some germ cells into adulthood. Spontaneous development is more commonly observed in patients with mosaicism with a 46, XX line [8].
In Mayer-Rokitansky-Kuster-Hauser syndrome or mullerian agenesis [1,3], the FSH, LH and oestrogen levels are usually normal and except for primary amenorrhoea. Puberty usually progresses normally. As was performed in all patients, renal ultrasound should be performed in all patients with mullerian agenesis because of the prevalence of associated developmental anomalies of the kidneys [9]. None of the women had developmental anomalies of the kidney.
Although Black and Govan [10] evaluated 20 patients with primary amenorrhoea by laparoscopy, chromosomal study and gonadal biopsy, Shearman [11] supported that laparoscopy or laparotomy could only be useful in the presence of inconsistency between the laboratory and clinical findings. For example, in case 3, transabdominal ultrasound revealed bilateral streak ovaries, and infantile uterus, but laparoscopy revealed absent uterus with normal ovaries and well developed bilateral corpus luteum. Their findings are comparable to our present case series.
Thus, in respect to this case series there is need to inform these patients about their problems including infertility and that ovulation induction in the future can be invoked using various fertility regimes [5]. The patient on the other hand should inform their intending husbands of their preexisting gynaecological problem before embarking on marriage. Hormone replacement therapy is essential and regimes exist for the induction of secondary sexual characteristics. Oestrogen should be used alone for about 2 years, and then 2–3 years of gradual introduction of progestogens thereby establishing normal breast growth over a time frame that is equivalent to normal [2]. Reproduction could be by assisted reproduction via ovum donation, in vitro fertilization and embryo transfer. The role of gestational surrogacy is invaluable. Counselling, assurance of female gender at the time of diagnosis, and emotional support through the treatment phase (vaginal dilatation and or vaginoplasty) are essential components in the management of patients with mullerian agenesis [3,9]. Very recently, successful pregnancy following uterine transplant has also been reported in cases of mullerian agenesis [12].
We reported a case series of primary amenorrhoea that underwent laparoscopy and dye tests in our center. Although reports regarding this gynaecological entity have been published, review of literature revealed only a few occurrences in our environment. The various options of evaluation and management of these cases including the adjuvant role of laparoscopy were discussed.
- Chang WY, Decherney AH (2007)Amenorrhoea. In: DeCherney AH, Nathan L (Eds). Current Obstetrics and Gynaecologic Diagnosis and Treatment. 10th Edition. Lange McGraw Hill, New York 926-936.
- van Dillen J, van der Honing A (2005) Primary amenorrhoea: three cases from a semi-rural Namibian hospital. Trop Doct 35: 186-188. [Crossref]
- Edmonds DK (2007) Primary Amenorrhoea. In: Edmonds DK (Ed). Dewhurst’s Textbook of Obstetrics and Gynaecology. 7th Edition. Blackwell Science, London, UK 369-376.
- Deshmukh MA, Vaidya PR, Motashaw ND (1978) Laparoscopy in primary amenorrhea. J Postgrad Med 24: 106-108. [Crossref]
- Timmreck LS, Reindollar RH (2003) Contemporary issues in primary amenorrhea. Obstet Gynecol Clin North Am 30: 287-302. [Crossref]
- Harland EJ, Damodaram M, Musaib-Ali L, Yoong W (2007) Primary amenorrhoea caused by congenital absence of the uterus. Arch Gynecol Obstet 275: 199-201. [Crossref]
- Aremu AA, Adetiloye VA, Ibitoye BO, Asaleye CM, Oboro VO (2006) Mayer-Rokitansky-Kuster-Hauser Syndrome: Two Cases of a Rare Non Hereditary Disorder in Siblings. The Internet Journal of Radiology 5: 1528-8404.
- Muran D (2007) Paediatric and Adolescent Gynaecology. In: DeCherney AH, Nathan L (Eds). Current Obstetrics and Gynaecologic Diagnosis and Treatment. 10th Edition. Lange McGraw Hill, New York 540-569.
- Mitan LAP, Slap GB (2002) Dysfunctional Uterine Bleeding. In: Neisntein L (ed). Adolescent Health Care: A practical Guide.4th Edition, Baltimore, MD, Lipincott Williams and Wilkins 966-972.
- Black WP, Govan ADT (1972) Laparoscopy and Gonadal Biopsy for Assessment of Gonadal Function in Primary Amenorrhoea. Br Med J 1: 672-675. [Crossref]
- Shearman RP (1968) A physiological approach to the differential diagnosis and treatment of primary amenorrhoea. J Obstet Gynaec Brit Cwlth 75: 1101-1104.
- Brännström M, Johannesson L, Bokström H, Kvarnström N, Mölne J, et al. (2015) Livebirth after uterus transplantation. Lancet 385: 607-616.


