Abstract
Background: Laparoscopic mini-gastric bypass (MGB) is gaining popularity because of favorable weight loss, co-morbidity resolution, simplicity and exit strategies. We evaluated the efficacy of the MGB in %EWL and resolution of type 2 diabetes (T2D) in different age-groups who had different durations of T2D. The successful end-result was set at HbA1c <6.5.
Methods: The study is a retrospective analysis of prospectively-collected data on 189 T2D patients who had undergone MGB from Jan 2007 to Dec 2014. Mean pre-operative age was 50.4 years (27-75 years). Mean duration of T2D was 12.75 years (6 months - 25 years). Mean pre-operative HbA1c was 10. 4% (6.9%-13.9%), with mean C-peptide 6.65 (0.24-13.05).
Results: Mean EWL was >90%. For the same length of bypass, the rate of %EWL was greater in the younger age group (27-40 years). T2D resolved in 95.1% of the patients (complete cessation of medication for T2D), with a significant improvement in the remainder. Although in few patients, who were on insulin, the requirement for exogenous insulin persisted, but the dose of insulin was reduced significantly. Those with shorter duration of T2D (6 months to 5 years) showed faster remission.
Conclusions: MGB was effective in both %EWL and improvement in glycemic control, thus leading to clinical resolution or improvement in T2D and its related complications. Nutritional deficiencies and gastroesophagealreflux were easily avoidable with proper technique. The simplicity, results and exit strategies make MGB a superior bariatric and metabolic procedure.
Key words
Mini-Gastric Bypass (MGB), Diabetes mellitus type 2 (T2D), Laparoscopic, Roux-en-Y gastric bypass(RYGB),Diabesity, Percent excess weight loss (%EWL), HbA1c, C-peptide, Fasting blood glucose (FBG)
Introduction
The increased global incidence of obesity and type 2 diabetes (T2D) have become two crucial health issues in developing and developed countries [1]. Increased body fat has been linked to numerous co-morbidities, such as dyslipidemia, cardiovascular disease, and T2D [2]. Lifestyle and environmental changes have led to an increased incidence of both obesity and diabetes [3]. Over the past two decades, the number of individuals with T2D has increased rapidly worldwide [4,5], and the term “diabesity” has been used to illustrate the close relationship between obesity and diabetes [6,7], and the vast majority of T2D individuals are obese [8,9]. Genetic susceptibility and obesity are the two most important risk factors for T2D [10].
Although lifestyle modifications and medical treatment is the mainstay for obesity and T2D, the last decade has proven that weight loss surgery is an effective solution, especially for morbidly obese T2D patients [11,12].
It is generally accepted that bypass operations have more powerful effects on T2D than gastric restrictive operations. Both Roux-en-Y gastric bypass (RYGB) and Mini-Gastric Bypass (MGB) act on the principle of restriction and malabsorption, but MGB is simpler with better outcomes, proven in a number of published comparative studies [13-15].
Materials and methods
The present study was conducted in a single center - Jammu Hospital, Jalandhar, India, with the data collected prospectively and analyzed retrospectively. The study group consisted of all patients with T2D who underwent MGB from January 2007 to December 2014 and had at least 6 months of follow-up. An informed consent was obtained from all the patients. T2D patients with a BMI of ≥ 30 kg/m2 were offered MGB after failure of conservative treatment, following complete evaluation by a multi-disciplinary team.
Surgical technique
The MGB is a mildly restrictive but importantly a malabsorptive operation, started in 1997 by Robert Rutledge [16]. MGB was performed using a 5-trocar technique, with the first stapler firing perpendicular to the lesser curvature distal to the crow’s foot, using a 45-mm blue cartridge. This was followed by vertical gastric division continuing proximally to the left of the angle of His (which was explicitly not dissected). Thus, a long gastric tube was created loosely against a 38-Fr bougie. The excluded part of the stomach remained in situ (Figure 1). Next, the jejunum was measured 200 cm distal to the ligament of Treitz, where an antecolic wide gastrojejunostomy (GJ) was performed using a 45-mm or 60-mm blue cartridge. The GJ anastomosis may be placed more proximally or distally, depending on the need for weight loss [17]. A long gastric conduit and a length bypass with a longer common channel (minimum 300 cm) avoided complications [14].
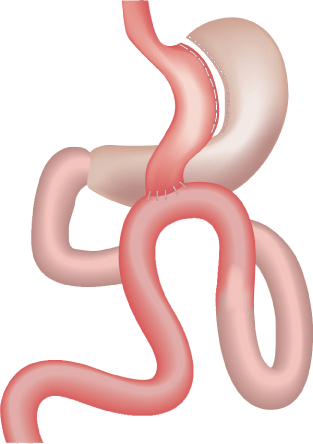
Figure 1. MGB: a lesser curvature channel, is constructed beginning distal to crow’s foot. This gastric channel is anastomosed to jejunum 200 cm distal to Treitz ligament, varied as indicated.
Data collection
The data consisted of 229 T2D patients, of whom 54. 6% (125) were female and 55. 4% (104) were male. Mean age was 51 (27 to 75) years, and mean BMI was 50 kg/m2 (30 to 70). The patients were further categorized as patients who were on insulin and patients who were on oral anti-diabetic medicines. Minimum duration of T2D was 6 months and maximum was 25 years. Data was collected from the in-patient and out-patient medical records, phone calls and electronic media. Data included pre-operative investigation, and post-operative outcome at 30 days, 3 months, 6 months, 1 year and yearly thereafter.
The database included pre-operative demographics and anthropometric features including BMI, weight, excess weight loss (EWL), fasting glucose, HbA1c, C-peptide level, type of anti-diabetic treatment and follow-up information. Morbidly obese patients, previously diagnosed as T2D but having normal HbA1c levels with metformin or anti-diabetic medications, were also included in the study. Patients were stratified into groups based on duration of T2D (≤5years, 6 to 15 years and 16 to 25 years), only on insuling or only on oral anti-diabetic medicines, and T2D without or with complications (retinopathy, neuropathy and nephropathy).
Pre-operative check-up
Pre-operative evaluation including history and physical examination, and nutritional and psychiatric assessment were performed on all patients. All patients were screened for T2D, and co-morbidities associated with T2D and obesity was recorded.
Outcome measures
The main parameters considered during the study were:
Weight Loss: Expressed as %EWL or change in BMI, recorded at every patient visit or through follow-up calls, and entered in the computer database.
Diabetes Control measured as:
- Change in Laboratory Outcomes: Blood glucose levels and HbA1c. HbA1c is a measure of the degree to which erythrocyte hemoglobin is glycosylated, expressed as percentage of total hemoglobin concentration. HbA1c provides an indication of average blood glucose ratio during the preceding 2-3 months. Normal values (5-6%) indicate very good glycemic control, 7-8% good glycemic control, 8-9% fair glycemic control, and >9% poor glycemic control. HbA1C was measured at 3-month intervals; for patients who could not come to the hospital, data was collected through phone or email via family physician.
- Change In Anti-diabetic Medication: The frequency and daily dose of anti-diabetic medicines varies with the severity of T2D, so it was used as an indicator for T2D status. The number of agents, strength and frequency of dose were used to assess improvement and remission of T2D (oral agents and insulin).
- Improvement In Diabetes-Related Co-Morbidities: After MGB, patients were clinically assessed for T2D improvement, including diabetic retinopathy, neuropathy and nephropathy.
- Impact On Nutritional Status: Because MGB is a bypass, careful monitoring of nutritional status by a team of nutritionists was important. Patients with longer bypass lengths had some nutritional concerns like hair loss, gallstone formation, anemia, vitamin-D deficiency, bone loss, and importantly protein deficiency – hypoalbuminemia[14].
The protocol which we followed to prevent nutritional deficiencies was bypass lengths limited to 200cm. For the super-obese (BMI >50), possible longer limb lengths were tailored [17],keeping in mind the nutritional status, compliance, age and family support. Vegetarians were encouraged to eat high protein diet, comprising tofu, soya bean and its products, legumes and grains, peanut butter, corn and sometimes whey protein supplements. For the non-vegetarian Indian population, protein deficiency was never seen, since the eggs, chicken and fish provided sufficient protein to avoid deficiency.
- MGB was offered to patients found to be committed after psychological counseling. Compliance is required for intake of vitamins and minerals, patients committed to follow-up, and ready to accept changes in lifestyle and eating habits.
Age and gender: Longer bypasses were avoided in age >55 years, to prevent nutritional deficiencies. It was found that heavy males, age <55 years withstood longer bypasses more comfortably. Females in the menstruating age group were counseled about iron intake.
Family support: Before surgery, counseling to patient and family by the team (bariatric surgeon, nutritionist, psychologist, family physician) was done. Satisfaction by all members of the team was the criteria for longer bypass if indicated; otherwise, bypass of 200 cm or less was not exceeded.
Statistical analysis
Data are presented as mean ± SD unless otherwise stated. Data before and after surgery were compared using Student’s t-test. A p-value <0.05 was considered significant.
Results
From Jan 2007 to Dec 2014, 229 diabetic patients underwent MGB. Complete follow-up information was achieved in 189 of the 229 patients (82.5%), and mean duration of follow-up was 51 months (minimum 6 months and maximum 96 months). The 40 patients with incomplete follow- up were excluded from the study, which included 12 patients with <6 months follow-up and 28 patients who could not be contacted despite multiple attempts, probably due to change of contact numbers and addresses.
The 189 patients with complete data consisted of 112 (59.3%) females and 77 (40.7%) males. Pre-existing diabetes-related complications were nephropathy 2.6% (5), retinopathy 0.5% (1) and neuropathy 1.1% (2) (Table 1).
Weight Loss: The mean %EWL was >90%. There was no relationship between EWL and prior duration of T2D. However, there was a relationship between EWL and age group. %EWL was faster in the younger age group (27-40 years) even for the same length of bypass. All patients achieved %EWL >75%. Weight loss did not correlate with severity of T2D (Table 2).
Diabetes control
Glycosylated hemoglobin: MGB had a marked effect on T2D. HbA1c markedly improved or returned to normal. Out of 189 patients, 180 patients achieved HbA1c levels between 5.0 and 6.2% without anti-diabetic medicine. Out of 50 patients who were on insulin, 9 achieved HbA1c levels of 8% and had to take additional anti-diabetic medicine to achieve HbA1c levels <6.5% (Table 2).
Change in usage of anti-diabetic medication: A significant reduction in usage of oral anti-diabetic medicine and insulin followed MGB. Patients who were taking multiple medicines for T2Dshifted to a single agent. The 9 patients who still had to take oral anti-diabetic medicines were those with a long history of uncontrolled diabetes and whose C-peptide levels were very low (<1) (Table 2). All the diabetic patients were put on metformin 500mg daily after MGB for a minimum of 1 year.
Improvement in diabetes-associated co-morbidities: Complete remission or improvements in diabetes-associated co-morbidities were noted in the majority of patients. All 5 patients with diabetic nephropathy showed significant improvement in renal function a few months after the MGB. The two patients with diabetic neuropathy showed some improvement. The patient with diabetic retinopathy who had very compromised vision showed improvement in eyesight (Table 2).
Discussion
The goal of this study was to determine the effect of MGB on T2D remission in obese and morbidly obese patients. Our results showed that there is a marked reduction in blood glucose levels and HbA1c after MGB. Biochemical and clinical analysis showed a diabetic remission rate of 95.23%, similar to results in other studies [18-20]. The magnitude of %EWL and resolution of T2D were independent factors of T2D remission, as diabetes went into remission even before the target %EWL. Patients with shorter duration of T2D showed earlier remission compared with patients with longer duration of T2D. Higher levels of stimulated C-peptide were associated with better outcomes in our study; higher levels of C-peptide had earlier and complete remission. Dixon et al. also found that %EWL and pre-operative duration of T2D were independent predictors of diabetes remission after gastric banding [21].
The younger age group showed a faster rate of weight loss compared with the older group. It may be due to high BMR in the younger age group and easy adaptability to the new milieu. Sugerman et al. found that younger age and %EWL were predictors of T2D resolution [22]. Younger age group, duration of T2D and higher β-cell mass were the predictors of remission in our study. Nine patients, who were on insulin pre-operatively, still required insulin post-operatively but in much reduced dosage. Associated microvascular and macrovascular co-morbidities (retinopathy, neuropathy and nephropathy) showed improvement. MGB can induce a significant and sustainable improvement in T2D and improve or halt the development of microvascular complications such as nephropathy. An improvement in neuropathy and retinopathy after bariatric surgery has been found by others [23-25].
We had previously found MGB to be a more effective and safe bariatric and metabolic procedure than sleeve gastrectomy and RYGB [14].
Conclusions
T2D in morbidly obese individuals is no longer an uncontrollable disease. A return to normal levels of glucose, insulin, HbA1c and ideal weight are attainable with MGB in the majority of obese diabetic patients. The quality of life improved in all the patients. MGB results in sustained weight loss and significant improvement in glycemic control, leading to clinical resolution or improvement in T2D and its related complications.
2021 Copyright OAT. All rights reserv
Disclosures
The authors have no conflicts of interest or financialties to disclose.
Acknowledgement
The authors thank Mervyn Deitel, MD, FASMBS, FACN, CRCSC for editorial advice. We thank Jashanreet Jammu,an MBBS student for assistance with data collection.
References
- Puska P, Nishida C, Porter D (2003) Obesity and overweight. World Health Organization report.
- Bray GA, Bouchard C. Handbook of obesity clinical applications, second edition.
- Zimmet P, Alberti KG, Shaw J (2001) Global and societal implications of the diabetes epidemic. Nature 414: 782-787. [Crossref]
- Amos AF, McCarty DJ, Zimmet P (1997) The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet Med 14 Suppl 5: S1-85. [Crossref]
- King H, Aubert RE, Herman WH (1998) Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care 21: 1414-1431. [Crossref]
- Astrup A, Finer N (2000) Redefining type 2 diabetes: 'diabesity' or 'obesity dependent diabetes mellitus'? Obes Rev 1: 57-59. [Crossref]
- Shafrir E (1996) Development and consequences of insulin resistance: lessons from animals with hyperinsulinaemia. Diabetes Metab 22:122-131. [Crossref]
- Halpern A, Mancini MC (2005) Diabesity: are weight loss medications effective? Treat Endocrinol 4: 65-74. [Crossref]
- Stumvoll M, Goldstein BJ, van Haeften TW (2005) Type 2 diabetes: principles of pathogenesis and therapy. Lancet 365: 1333-1346. [Crossref]
- Scheen AJ (2003) Pathophysiology of type 2 diabetes. Acta Clin Belgica 58: 335-351. [Crossref]
- Kim Z, Hur KY (2011) Laparoscopic mini-gastric bypass for type 2 diabetes: the preliminary report. World J Surg 35: 631-636. [Crossref]
- Brethauer SA, Aminian A, Romero-Talamás H, Batayyah E, Mackey J, et al. (2013) Can diabetes be surgically cured? Long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Ann Surg 258:628-636. [Crossref]
- Milone M, Di Minno MN, Leongito M, Maietta P, Bianco P, et al. (2013) Bariatric surgery and diabetes remission: sleeve gastrectomy or mini-gastric bypass? World J Gastroenterol 19: 6590-6597. [Crossref]
- Jammu GS, Sharma R2 (2016) A 7-Year Clinical Audit of 1107 Cases Comparing Sleeve Gastrectomy, Roux-En-Y Gastric Bypass, and Mini-Gastric Bypass, to Determine an Effective and Safe Bariatric and Metabolic Procedure. Obes Surg 26: 926-932. [Crossref]
- Quan Y, Huang A, Ye M, Xu M, Zhuang B, et al. (2015) Efficacy of Laparoscopic Mini Gastric Bypass for Obesity and Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Gastroenterol Res Pract 2015: 152852. [Crossref]
- Rutledge R (2001) The mini-gastric bypass: experience with the first 1,274 cases. Obes Surg 11: 276-280. [Crossref]
- Lee WJ, Wang W, Lee YC, Huang MT, Ser KH, et al. (2008) Laparoscopic mini-gastric bypass: experience with tailored bypass limb according to body weight. Obes Surg 18: 294-299. [Crossref]
- Musella M, Apers J, Rheinwalt K, Ribeiro R, Manno E, et al. (2016) Efficacy of Bariatric Surgery in Type 2 Diabetes Mellitus Remission: the Role of Mini Gastric Bypass/One Anastomosis Gastric Bypass and Sleeve Gastrectomy at 1 Year of Follow-up. A European survey. Obes Surg 26: 933-940. [Crossref]
- Kular KS, Manchanda N, Cheema GK (2016) Seven Years of Mini-Gastric Bypass in Type II Diabetes Patients with a Body Mass Index <35 kg/m(2). Obes Surg 26: 1457-1462. [Crossref]
- Robert M, Ferrand-Gaillard C, Disse E, Espalieu P, Simon C, et al. (2013) Predictive factors of type 2 diabetes remission 1 year after bariatric surgery: impact of surgical techniques. Obes Surg 23: 770-775. [Crossref]
- Dixon JB, Dixon AF, O'Brien PE (2003) Improvements in insulin sensitivity and beta-cell function (HOMA) with weight loss in the severely obese. Homeostatic model assessment. Diabet Med 20: 127-134. [Crossref]
- Sugerman HJ, Wolfe LG, Sica DA, Clore JN (2003) Diabetes and hypertension in severe obesity and effects of gastric bypass-induced weight loss. Ann Surg 237: 751-756. [Crossref]
- Pérez G, Devaud N, Escallop A, Downey P (2006) Resolution of early stage diabetic nephropathy in an obese diabetic patient after gastricbypass. Obes Surg 16:1388-1391. [Crossref]
- Kotoula MG, Koukoulis GN, Zintzaras E, Karabatsas CH, Chatzoulis DZ (2005) Metabolic control of diabetes is associated with an improved response of diabetic retinopathy to panretinal photocoagulation. Diabetes Care 28: 2454-2457. [Crossref]
- Müller-Stich BP, Fischer L, Kenngott HG, Gondan M, Senft J, et al. (2013) Gastric bypass leads to improvement of diabetic neuropathy independent of glucose normalization: results of a prospective cohort study. Ann Surg 258: 760-766. [Crossref]

