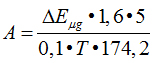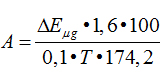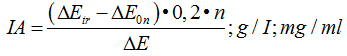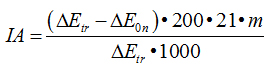Abstract
A new theory of grippe pathogenesis with participation of proteinase-inhibitory system has been offered. It has been established that purification and concentration of grippe viruses by different methods did not release the virus from cellular enzymes. At the experimental animals infecting with the virus of grippe, disturbance of enzyme-inhibitory balance took place, especially during first hours after the animals being infected. From the lungs of healthy mice, they have got six isoforms of trypsin-like proteinases. To all of them they got antiproteinase immune sera and have treated the experimental animals. It was antiserum to the third isoform that has prevented the experimental animals fatality.
Introduction
In the pathogenesis of viral diseases the interaction of virus and cell is under-investigated. Penetration of a virus into a healthy cell with virus’s obligatory deproteinization is of top importance here. While viruses deproteinization is studied insufficiently. First of all it refers the mechanisms of grippe virus’s penetration into the cell of mammals, including a human being. In this respect win 1983 we offered a new theory of grippe pathogenesis with proteinase-inhibitory system (PIS) participation [1,2]. The difficulties of new antiviral drugs creation rise from specific biological properties of viral diseases pathogens. The recent findings of biochemistry and molecular biology have highlighted some peculiarities of virus’ reproduction and provided new approaches for a directed interference into the cycle of viral reproduction [3,4].
The objective
to examine the state and role of antiproteinase systems of virus and recipient in the development of influenza infection and obtained fundamentally new medicinal preparations based on of trypsin – like proteinases inhibitors.
The tasks
- To learn the proteinases’ and their inhibitors role at different, especially early stages, of grippe development.
- To eliminate and purify proteinase and its inhibitor from the lungs of healthy and infected by grippe mice.
- To investigate the protective properties of cellular inhibitor at infection of the animals with lethal dose of grippe virus.
Results
At early 1980 when purified and concentrated different races of grippe viruses to obtain polyvalent anti- grippe vaccines we met with impossibility to release influenza’s virus from protolithic activity. To solve this problem we have improved the methods of purification but nevertheless we failed to purify influenza virus from protolithic activity [5].
Analysis of purified preparations of influenza virus showed that ultracentrifugation does not release influenza’s virus from protolithic activity and in saccharose’s gradient (15-60%) it distinctly got into several isoforms (Table 1).
Expire-ments |
№ of sugar gradient fraction |
% of sucrose |
Proteinase before neutralization, mkg/arg per
min in 1 ml |
Proteinase after
neutralization, mkg/аrg per
min in 1 ml |
HA before neutralization in
0.1 ml |
HA after
neutralization
in 0.1 ml |
1.Influenza
Virus |
1 |
5 |
1.42 |
0 |
0 |
1:2 |
2 |
15 |
32.0 |
0 |
1:8 |
1:2 |
3 43
4 |
32
42 |
8.5
29.8 |
00
0 |
1: 16
1:2048 |
1: 8
1:2048 |
5 |
49 |
3.9 |
0 |
1:1048 |
1:512 |
6 |
52 |
32.9 |
0 |
1:64 |
1:64 |
7 |
55 |
32.2 |
0 |
1:64 |
1:64 |
8 |
57 |
6.04 |
0 |
1:2 |
1:2 |
2.Influenza
Virus |
1
2 |
3
11 |
1.3
12.0 |
0 |
0
1:2 |
0
1:2 |
3 |
24 |
7.28 |
0 |
1:16 |
1:16 |
4 |
24 |
9.42 |
0 |
1:16 |
1:16 |
5 |
38 |
84.6 |
0 |
1:512 |
1:2048 |
6 |
42 |
23.28 |
0 |
1:2048 |
1:1024 |
7 |
46 |
10.8 |
0 |
1:1024 |
1:512 |
8 |
48 |
7.02 |
0 |
1:32 |
1:32 |
3.CAM |
1 |
6 |
0.05 |
0 |
0 |
0 |
2 |
17 |
0.101 |
0 |
0 |
0 |
3 |
27 |
0.037 |
0 |
0 |
0 |
4 |
29 |
0.059 |
0 |
0 |
0 |
5 |
37 |
0.080 |
0 |
0 |
0 |
6 |
41 |
0.143 |
0 |
0 |
0 |
7 |
49 |
0.064 |
0 |
0 |
0 |
8 |
53 |
0.128 |
0 |
0 |
0 |
9 |
56 |
0.108 |
0 |
0 |
0 |
Table 1. Protamine-shifting and hemagglutinating activity of influenza virus in fractions of sugar gradient to neutralization and after neutralization by immune (CAM) rat sera
CAM – chorionallantoic membrane; HA – hemagglutinating activity.
The results obtained allowed us to conclude that serine-containing proteinase of trypsin-like type of cellular origin with molecular heterogeneity is associated with influenza virus [6,7].
System of proteinases and inhibitors is presented in the body by a vast group of proteins. Inhibitors of protolithic enzymes have a role of the constant level of corresponding enzymes and are in a constant dynamic equilibrium with the latter. Disturbance between enzymes and inhibitors is of importance for the development of pathological processes.
Our researches show that the level of proteinase activity and activity which inhibits proteinase is in equilibrium in the lungs and blood serum of uninfected animals and the latter disturbances at infection by the virus of influenza A [8].
At communicable process the deepest changes have place during first hours after introduction of infection. Thus, in 6 hours after contamination the amount of proteinase in lungs and blood serum of the infected animals reduces while inhibiting activity increases. The cells contaminated by influenza virus induce appearance of inhibitor both in lung tissue and in blood serum. So, lung inhibitors are an organ “first line of resistance” at the action of various flu strains [9].
6 isoforms of trypsin-like proteinase were extracted from the lungs of the healthy mice and 8 isoforms –from the infected animals by ion-exchange chromatography. Their specific proteolytic activity vastly increased compared to the source material. The proteinase isoforms’ obtained had a wide range of substrate specificity and were able to hydrolyse substrates of both natural and synthetic origin (Table 2).
N of fraction |
N of isoform |
Specific proteolic activity per mg of protein |
% of proteinase outcome |
% of purification by protein |
33 |
I |
4.285 |
2.09 |
96.8 |
53 |
II |
83.75 |
5.84 |
99.07 |
65 |
III |
22.42 |
2.703 |
98.38 |
75 |
IV |
40.00 |
6.279 |
97.92 |
121-130 |
V |
32.6 |
136.74 |
99.98 |
161-189 |
VI |
0.787 |
421.74 |
64.90 |
Table 2. Purification DEAE –cellulose-53 of lung trypsin-like proteinase of healthy mice
Antiproteinase hyper immune rat sera were obtained to both isoforms of trypsin-like proteinases [10]. In studies of the protective properties of antiproteinase sera and serum of healthy rats on the white mice infected intranasal with the lethal dose of influenza virus A/PR/8/34 (IV passage) it was found that 100% of control mice fatality took place in 4-5 days. The animals to which they injected healthy rats serum intranasal 6 times, died on the 7th day. In the treatment of mice with the pools of immune sera I, II, IV, V and VI groups the animals mortality rate was reduced and lethality came much later than in the control group. 20% of the animals recovered (Table 3).
N
of
gr.
of
mice |
Isoform of proteinase |
Sera group |
Terms after infecting, hours and days |
6 h |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
14 |
Survived |
% of survive |
1. |
I |
I |
0/10 |
0/10 |
0/10 |
010 |
0/10 |
2/8 |
0/8 |
2/6 |
2/4 |
2/2 |
0/2 |
0/2 |
2 |
20 |
2. |
I |
II |
2/8 |
2/8 |
0/6 |
4/2 |
0/2 |
0/2 |
0/2 |
0/2 |
0/2 |
0/2 |
0/2 |
0/2 |
2 |
20 |
3. |
II |
III |
0/10 |
0/10 |
2/8 |
0/8 |
0/8 |
2/6 |
0/6 |
2/4 |
0/4 |
0/4 |
2/2 |
0/2 |
2 |
20 |
4. |
III |
IV |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
2/8 |
0/8 |
0/8 |
2/6 |
0/6 |
0/6 |
0/6 |
6 |
60 |
5. |
IV |
V |
0/10 |
0/10 |
2/8 |
2/8 |
2/6 |
2/4 |
2/2 |
0/2 |
0/2 |
0/2 |
0/2 |
0/2 |
2 |
20 |
6. |
V |
VI |
0/10 |
0/10 |
5/5 |
3/2 |
2/0 |
0/0 |
0/0 |
0/0 |
0/0 |
0/0 |
0/0 |
0/0 |
0 |
100 |
7. |
VI |
VII |
0/10 |
0/10 |
0/10 |
7/3 |
1/2 |
1/1 |
0/1 |
0/1 |
0/1 |
0/1 |
0/1 |
0/1 |
1 |
10 |
6. |
Sodium chloride solution |
|
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
10 |
100 |
7. |
Serum-free virus |
|
0/10 |
0/10 |
0/10 |
0/10 |
2/8 |
6/2 |
2/0 |
0/0 |
0/0 |
0/0 |
0/0 |
0/0 |
0
|
0 |
8. |
Healthy rats serum |
|
0/10 |
0/10 |
0/10 |
0/10 |
2/8 |
3/5 |
0/5 |
5/10 |
5/0 |
0/0 |
0/0 |
0/0 |
0
|
0 |
9. |
Immune serum IVgr., virus-free (toxicity) |
|
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
0/10 |
10 |
100 |
Table 3. Influence of antiproteinase immune sera on survival of mice under infection with lethal dose of influenza virus A/H1N1/PR/8/34
Note: 1. numerator – number of mice died; 2 – denominator – number of mice in the experiment.
The most effective was the fourth pool of immune serum to the IIIrd isoform. In its presence 60% of infected mice survived and on the 14th post-infection day in the blood serum and in the lungs we did not detect either hemagglutinin, no infectious virus. Immune serum to isoform VI did not protect mice from death at all, although isoform III differed from isoform VI by only one protein with molecular mass 32 kDa.
From the lungs of healthy mice, we have isolated an inhibitor of trypsin-like proteinases with molecular mass 47.5 kDa, with a high degree of purity and small amount of impurities. We have designed and patented a technique of procedure and decontamination of trypsin-like proteinases [11,12]. The inhibitor isolated is a like α1- inhibitor of human blood serum (m.m. 48-55 kDa) and egg-yolk trypsin (m.m. 49 kDa) but is unlike the inhibitor isolated from the lungs of cattle (inhibitor of Kunitz-Northrop type) with molecular mass 65kDa. In studies of its effect on trypsin-like proteinases isoforms by tube test it has been revealed that it suppresses activity of practically all isoforms, excluding the 4th (41.8%) and the 8th (28.3%).
In our researches it has been revealed that the cellular inhibitor suppressed the development of grippe virus in the chicken’s embryos and development of infectious and hemagglutinating activity and formation of the total protein. At the same time inhibitor of trypsin-like proteinases extracted from the lungs of previously viremic mice had no such a property. At our further researches for the treatment of grippe infection in animals we used inhibitor extracted from the lungs of healthy animals [13]. Administration of this inhibitor to the previously viremic by lethal dose of grippe mice reduced the percentage of their deaths because of HA decomposition inhibiting at virus’s reproduction in lungs, arresting of the process generalization and due to the prevention of increase of proteolysis in lungs [14], precaution of aero-hematic barrier and some hyper reaction of local protection (Table 4).
N and name of
group of animals (white mice) |
Number of animals in the group
|
Dose of influenza virus
5 LD50 |
Dose of inhibitor, µg on a mice, one injection |
Number of animals |
% of animals protected from virus |
Dies |
Survived |
1. Influenza virus |
40 |
10-3 |
- |
40 |
- |
0 |
2. Influenza virus +trypsin, crystal. |
40 |
10-3 |
18 µg |
40 |
- |
0 |
3. Influenza virus + inhibitor from healthy lungs |
40 |
10-3 |
18 µg |
7 |
33 |
82.5 |
4. Cellular inhibitor |
40 |
10-3 |
18 µg |
- |
40 |
100 |
5. Trypsin, crystal |
10 |
- |
18 µg |
- |
10 |
100 |
6. Phosphate buffer |
10 |
- |
0.2 ml |
- |
10 |
100
|
Table 4. Action of cellular inhibitor of trypsin-like proteinase of the survival rate of mice infected with lethal dose of influenza virus А/PR/8/34
Discussion
One of the most important stages of the development of many viruses in the host organism is their introduction in the cell after preliminary deproteinization. Regulation of this development of virus is one fundamental principles of their reproduction. Induction or intake of inhibitor of virus proteolytic activation is one of the promising ways of viral diseases treatment, including influenza.
Methods
Strains of influenza virus:
A/PR/8/34 (H1N1), grown on a 9-day chicken embryos, were obtained at the D.I. Ivanovsky Research Institute of Virology, Academy of Medical Sciences of Russia and strain AO/32 (H1N1) - from the Influenza Research Institute of St. Petersburg, Russia; white mice and hybrids; chicken embryos; white rats, Wistar line.
Virological analysis
Infection and accumulation of influenza virus A/PR/8/34(H1N1) on chicken’s embryos. Adaptation of influenza virus A/PR/8/34 to white mice. Four passages of influenza virus has been done. A fatal dose of influenza virus equals to 5 LD50 has been obtained. The choice of the dose of trypsin-like proteinase inhibitor for the treatment of white mice infected with IAV.
Concentration and purification of influenza virus
Influenza virus AO/32 (H1N1) with infectious titers 7.0 log EID50/0,2ml and hemagglutinin (HA) -1:256. To obtain influenza virus preparations we used 10-11-day chicken embryos. The virus was accumulated by infecting chicken embryos in a volume of 0.2 ml, diluted to 10-3 with infectious material. Infected chicken embryos were incubated for 48 hours at +36°C. Then they were cooled for 18 hours at +4°C and then the virus-containing fluid was collected, purified and concentrated with centrifugation.
Concentration and purification of influenza virus was done in as follows: virus-containing liquid was consecutively centrifuged with 6 000 R.P.M. during one hour at +4°С on centrifuge CLR-1 (Ukraine) for purification from components of tissue and erythrocytes. Then 30 min at 10,000 R.P.M. at +4°С on centrifuge CLR-1 (Ukraine) to remove the main mass of cellular proteins. After that virus was precipitated at 2000 R.P.M. during an hour on centrifuge CLR-1 (Ukraine) through 20% layer of sucrose for more complete purification of virus. Purified and concentrated virus was additionally purified with speed centrifugation in sucrose density gradient (15-60%) on centrifuge VAK-602 in backet-rotar at 28 000 R.P.M. during four hours. Influenza virus which was in the zone 37-45% of sucrose was reultracentifugated at 33 000 R.P.M. in rotor SV-40, ultracentrifuge Spinko, three hours.
In the preparations obtained and eluates of fractions the content of proteins in O.H. Lowry [15] method was controlled after dialysis at all stages. As well as content of HA in routine method with 1% solution of chickens embryo erythrocytes and the presence of proteolitic activity in K. N. Veremeyenko method [16] in S. V. Vovchuck’s [17] modification.
Study of trypsin-like proteinasis role in the development of viral infection in mice
In the present work we use influenza virus A/PR/8/34 (H1N1) adopted to lung tissue of white mice; virus’ infectious titer was 7.0 log EID50/0,2 ml and HA titer 1:128. Outbred white mice with mass of 16-17 gr were taken in the experiment. Contamination of the animals was done at a light ether intranasal anesthesia, volume 0.05 ml at the dilution 10-3, which corresponded influenza infectious dose 5 LD50. This dose afforded 100% animals’ fatality on the 6th day after contamination. The animals were killed and their lungs taken off (5 mice in each group) in 15 and 30 min; 1 hour; 6 hours, etc. and later on 1; 2; 3; 4; 5; 6 days after contamination. Concurrently, non-infected mice were killed and their lungs extracted (5 animals in each group) at the same terms. Blood was taken as well. Lungs were washed in cold 0.01M phosphate buffer pH 7.5 twice, grinded in a cold mortar, suspended in phosphate buffer (1 ml for one lung), homogenized with ultrasound at the mode 7 on High Intensity Ultrasohie Procession, Chicago Cole Parmel (USA), centrifugated at 10 000 R.P.M. on the centrifuge RS-5 (Sorvall Instruments, Rotor SS-34), during 1 hour, temperature +4°С. Supernatant and blood serum was used for determination infectious, proteinase and proteinase inhibiting activities. Virus infectious titer in the lungs of infected mice and allantois liquid was determined by contamination 9 -10 days chicken embryos and expressed it in log EID50/0,2 ml.
Determination of trypsin inhibitor activity by casein’s hydrolysis was done in K. M. Veremeyenko method, modified by A. P. Levitsky [18].
Learning of virus influence on proteinase-antiproteinase activity
Influence of influenza virus on the dynamics of proteinase and inhibiting activity has been investigated at multiplication of virus in chicken embryo. Virus А/H1N1/PR/8/34, adopted to chicken’s embryo with HA titer 1:128 was used for contamination of 11-day chicken embryos in two doses: a large dose (at the dilution 10-1, that corresponded to 2,5-2 LD50) and a small one (at the dilution 10-6, that corresponded 1 LD50).
Virus-containing allantois liquid was taken in 15 and 30 min., 1 hour, 24, 48, 72, 96 and 122 hours after contamination. Three embryos were used for each period. Uninfected chicken embryos were used as a control. Allantois liquid was taken in them in 15 and 30 min., 1 hour, 24, 48, 72 and 122 hours (three embryos for each term) and learnt the same indexes as in contaminated material. In the material selected we learnt the presence of proteinase, inhibiting and infectious activity, HA and protein.
Biochemical analysis
We used chromatographic methods for extraction and purification of inhibitor from the wastes of sera-manufacturing industry. Protein determinations was done in O. Lowry method, determination of trypsin inhibitor activity by casein’s hydrolyse in K. M. Veremeyenko method, modified by A. P. Levitsky. Electrophoretic analysis was done in U.K. Laemmli method [19].
Extraction and purification of trypsin – like proteinases
Trypsin – like proteinases have been extracted from the lungs of the healthy mice (100 items for an experiment) and also from the lungs in 72 hours after contamination with influenza virus A/H1N1/PR/8/34 (100 items for an experiment). The experiment was performed with the mice of the line «Balb/с», weighted 16-18 gr. Contamination of the animals with the virus of influenza was performed under light ether anesthesia in the volume 0.05 ml in the dilution 10-6, which corresponds virus infectious dose 1 LD50.
In 72 hours after the animals’ contamination the animals were killed and their lungs were extracted. Parallels with it blood was taken. The lungs were shredded with sissors, then homogenized in a mortar, processed with ultrasound at 18 kHz, 75 “on the device Soniprep 150 MSE. The whole work was done at the temperature +4°С. Homogenate was centrifuged at 10,000 R.P.M. during 1 h at centrifuge RC, temperature +4°С. Supernatant I was freezed, 1% Triton X-100 was added to the precipitate and left in the refrigerator till morning.
Next day the precipitate was processed with ultrasound once more and centifugated at the same regimes. Supernatant II was taken. Both supernatants (I and II) were joined later on for purification.
Purification of the material dialyzated was done by ion-exchange chromatography on DEAE-cellulose-32, device ZKB - 2023, Minicololab (Broma). Colomn’s height was 18 cm, diameter 1.75 cm. The extent of purification was controlled by proteins and protease’s activity. Protein was determined by O. Lowry’s method and protamine-splitting activity by K. Veremeyenko method.
Fractions with high indexes of protease were purified additionally by dialysis, then dried by liophilization on Hetocite Heto (Denmark). Extracted and purified isoforms were used for immunization of the animals. At all stages of isoforms of trypsin-like proteinase purification, in all fractions determination of proteinase, inhibiting, hemagglutination activity and total protein was carried out, 19 biological repetitions were done.
Production of hyperimmune antiproteinase sera
To obtain sera we used white rats of Wistar line weighed 170-200 gr.
White rats were immunized four times, once per week with each isoform of trypsin-like proteinase extracted from the healthy and infected lungs of mice with a complete Friend’s adjuvant. Each rat got 560 units of protein and 890 units of proteinase. Total pick up of hyperimmune sera was done in 7 days after the last immunization.
Extraction and purification of trypsin inhibitor from lungs of mice
Extraction of inhibitor was done by the method used for proteinases extraction. Trypsin inhibitor purification was done by ion-exchange chromatography on DEAE–cellulose, gel-filtration – on Sephadexes G-15 and G-50, by affine chromatography on trypsin – sepharose 4В. In the last-mentioned case trypsin was covalently perfected in 0.05 М tris- НС1 buffer рН 7.6. Desorption was done consequently with buffer solutions, containing 1.0 М NaCl, 8 М urea and 0.2 М КС1-НСl рН 2.0 solution. Trypsin’s inhibitor in fractions was determined by deceleration of benzoyl-arginine-p-nitroanilide hydrolysis by crystalline trypsin (A.P.Levitsky, 1979).
Determination of trypsin-like proteinases activity by protamine hydrolysis
Qualitative reaction to arginine, forming at the hydrolysis of protamine and histones, and which does not precipitate with 20% CCl3COOH, forms the basis the method.
Reagents.
- 1.0 % solution of protamine sulphate on 0.1 M phosphate buffer pH 7.5.
- 20 % solution of CCl3COOH.
- 0.004 % solution of oxychinoline. 100 mg of oxychinoline was diluted in 50.0 ml of spirit of wine. The working solution was prepared by 50 times soluted with aqua distillate initial solution just before determination.
- 10.0 % NaOH.
- 10.0 % solution of NaBrO. 1.0 gr. Br (0.3 ml) was brought up to 100 ml with cooled to 0º C 5.0% solution NaOH.
- 40.0% solution of urea.
Procedure of determination. To the mixture, containing 0.2 ml of protamine sulphate and 0.5 ml 0.1 М phosphate buffer рН 7.5, 1 ml of enzymatic solution was added. The samples were incubated 150 min on the water bath at +38 °С. The reaction was stopped by 0.9 ml of 20.0 % CCl3COOH. The content of the tube was mixed and centrifuged 15 min at 6 000 R.P.M. 10 ml of supernatant was got over into ice-cooled tubes. 1.0 ml of oxychinolone solution, 1.0 ml of 10% NaOH and 0.2 ml of NaBrO was added in succession. The tubes were agitated and in 15 min 1.0 ml of 40,0 % urea was added. 1.0 ml of cooled distillated water was added and in 5 min the activity was checked at 508 ml (blue-green light filter). To the contral sample 0.8 ml of 20% CCl3COOH was added to 0.8 ml of protamine sulphate and phosphate buffer before.
0.5 ml 0.1 M of phosphate buffer pH 7.5; 0.1 ml of the solution under study; 0.8 ml CCl3COOH (20%). 0.2 ml 1% solution of protamine sulphate on phosphate buffer we added to the control sample.
Spectrophotometer was maintained by the control to reagents – instead of 1.0 ml of supernatant liquid 1.0 ml of 10.0% of CCl3COOH was added in the sample. The further determination was similar to that in the experimental sample.
Enzymes activity was determined with the following formula:
| Homogenate of lungs |
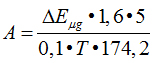 |
| Blood serum |
2021 Copyright OAT. All rights reserv
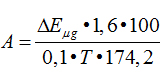 |
| Total formula |
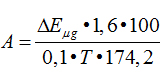 (modified by S.V.Vovchuk) (modified by S.V.Vovchuk) |
where, А – activity of enzyme in u/ml. A unit of activity is equal to an amount of enzyme causes formation of 1 micromole of arginine per 1 min of incubation; ∆Е – extinctions difference between experimental and control sample; n – dilution of enzymatic solution; 1.6 – the total volume of sample; 2 and 5 – recalculation for the whole mixer under intubation;100 – calculation on 100 ml of serum; 0.1 – volume of enzymatic solution; Т – time of incubation, min; 174.2 – molecular mass of arginine; К – coefficient of conversion of extinction amount to micromole of arginine.
For conversation of extinction to arginine’s mcmol we plotted a calibrated curve against standard solution of arginine.
Method of determination of inhibitor activity
Determination of proteinases inhibitors in lungs homogenates, blood serum, and allantoic liquid was done by casein’s method offered by A. P. Levitsky.
To put 0.2 ml of supernatant into new glass tubes.
To add 2 ml of reagent A and 2 ml of Folin’s reagent. Contact – 30 min at room temperature. Analyze at spectrophotometer.
Calculate of inhibiting activity (IA)
| Serum: |
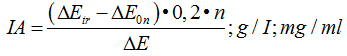 |
| Tissue: |
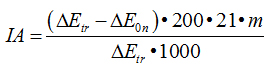 |
where: ∆Еtr - extinction of the sample with trypsin; n - dilution of the solution with serum; 0.2 – trypsin’s concentration, mg/ml; m – dilution of inhibitor’s solution; 200 – the amount of trypsin in 1 ml (200 mkg); 21– ratio of tissue’s charge to extragent, weight 100 mg. per 2 ml; 1000 – recalculation coefficient against 1 gr of tissue; I - соntent of inhibitor per 100 ml; 1 - a unit corresponding to 1 gr of crystalline trypsin; Е0n - extinction of the sample with the mixture trypsin + inhibitor.
Statistical analysis
The results of the investigations carried out have been processed with the programme «Microsoft®Excel».
References
- Divocha VA, Mikhalchuk VN, Gozhenko AI (2009) Molecular-and-biological substantiation of antiproteinase therapy of influenza. J Acad Med Scien Ukr 15: 19-21.
- Divocha VA, Mikhalchuk VN, Gozhenko AI (2009) Trypsin-like proteinase and its inhibitors in vaccines and immunobiological preparations of blood. J Acad Med Scien 15: 609-25.
- Savinova OV, Pavlova NI, Boreko YI (2008) Individual and complex use of new derivatives of betulin and remantadin for inhibition influenza virus reproduction. Modern Problems of Humans Infectious Pathology: Collected Scientific Works. Minsk (Belorussia): Belprint 1: 137-41.
- Shevchenko Y, Burtseva Y, Ivanova N (2007) Specific ant influenza chemical preparations, substantiation of their use for prophylaxis and treatment in Russia: Urgent Problems of Infectious Pathology and Vaccine Prophylaxis in Children, 13-14 December, Moscow: Abstracts, 36-38.
- Divocha VA, Degtiarenko VI, Zevakov VF (1983) Cellular proteinase of influenza virus: The 2nd Congress of Infectionists of Ukraine: Abstracts. Kiev, 36-38.
- Divocha VO (1998) Cellular components associated with virus of influenza. Odessa Med J 2: 8-10.
- Divocha VO (2000) Changes in chicken embryo at the action of influenza virus. Odessa Med J 2: 100-105.
- Divocha VA, Mikhalchuk VN, Gozhenko AI (2012) Dynamics of proteinase inhibitors, and the first phase of destruction of the body of white mice with experimental influenza virus. J Acad Med Scien Ukr 1: 133-136.
- Divocha VA, Mikhalchuk VN, Gozhenko AI (2011) Biological substantiation of antiproteinase therapy of influenza virus. Odessa: ART-V, 316 p.
- Divocha VA, Sova YG, Mikelashvili MT (1995) Protective role of antiprotease immune sera at experimental influenza. I.I. Mechnikov’s Ideas and the Development of Modern Natural Sciences: Abstracts of Scientific Conference, 28-30, November, Kharkov, 102-103.
- Divocha VO, Sova YG, Mihkalchuk BM (2001) Studying of physical-and-chemical properties of isoenzymes of trypsin-like proteinases. Med Chem 3: 31-34.
- Divocha VO (1998) The method of inhibitor of trypsin-like proteases obtaining: Patent N 23548 A (Ukraine), IPC6 A61K25/00.
- Divocha VO, Mikelashvili MT, Mikhalcuk VM (2001) The action of inhibitor of trypsin-like protease on influenza infection at the conditions of experiment. Infect Dis 2: 35-39.
- Divocha VO (2001) Inhibitor of trypsin-like proteases as antiviral remedy: Patent N 37324 A (Ukraine), IPC6 F61R31/14.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265-275. [Crossref]
- Veremeenko KH (1980) Enzymes in otolaryngologists. Kiev: Health, 147 p.
- Vovchuk SV (1979) Determination of activity of proteolytic enzymes in the grain cereals. Biochemical methods of breeding material Odessa 15: 69-74.
- Levitsky AP (1979) Methods for determination of trypsin inhibitors. Biochemical Methods of breeding material: Coll. Resear. Works Odessa 15: 68-73.
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680-685. [Crossref]