Abstract
Sarcomas are rare mesenchymal malignancies with diverse clinical and pathological features. Although the use of adjuvant chemotherapy for sarcomas leads to remarkable clinical benefits, novel anticancer drugs are required for better clinical outcomes in patients with sarcomas. Aberrant regulation of many tyrosine kinases drives the defective signaling pathways in sarcomas. We examined the inhibitory effects of three drugs on signal transduction pathways in sarcomas, using a panel of 20 sarcoma cell lines with different histological origins. The effects of BIBF 1120 on the triple receptor kinase pathway, BI 860585 on the mTOR signaling pathway, and BI 836845 on the insulin-like growth factor (IGF)-induced signal transduction pathway were investigated. Osteosarcoma, rhabdomyosarcoma, synovial sarcoma, and Ewing sarcoma cell lines were used for the study. Our findings indicated that BIBF 1120 may preferentially inhibit the viability of synovial sarcoma cell lines, BI 860585 may have inhibitory effects on the viability of sarcoma cells with different histological features, BI 836845 may have specific inhibitory effects in Ewing sarcoma, and IGF-1 receptor may be a predictive biomarker of sarcomas. However, further investigations on the molecular mechanisms involved in the responses to treatments with the three inhibitors are necessary for developing novel therapeutic strategies against sarcomas.
Key words
BIBF 1120, BI 860585, BI 836845, sarcoma, tyrosine kinase inhibitor
Introduction
Sarcomas are mesenchymal malignancies with different histological backgrounds. The clinical features of sarcomas range from curable to metastatic tumors, which result in short-term survival. Although surgical resection is the treatment of choice for the localized disease, 50% of high-grade sarcomas tend to recur [1]. The use of adjuvant chemotherapy, which started in the early 1970s, led to remarkable improvements in the outcome of sarcoma patients. For example, the survival rates of patients with localized osteosarcoma [2-6] and Ewing sarcoma [7-9], both of which are sensitive to chemotherapy, were improved from approximately 20% to 70% by adding multiagent chemotherapy to the surgical removal of the tumors. However, intensified chemotherapy has not been noted to substantially improve the clinical outcomes of osteosarcoma [10,11] or Ewing sarcoma [12,13]. Moreover, in sarcomas that do not respond well to chemotherapy, the role of adjuvant treatment remains a subject of discussion because its potential benefit does not exceed its side effects. These notions drove the development of a novel therapeutic strategy such as targeted one [14].
The advent of targeted drugs has considerably contributed to the recent trends in sarcoma treatment [15]. The US Food and Drug Administration (FDA) has approved the following targeted drugs for the treatment of soft tissue sarcomas: imatinib mesylate [16], pazopanib hydrochloride [17], eribulin mesylate [18], and trabectedin [19]. Immunotherapeutic approaches have also been introduced for the treatment of sarcomas [20]. Considering the substantial number of FDA-approved targeted drugs for treating other malignancies, a higher number of targeted drugs will be adapted to sarcoma treatments.
In this study, we examined the inhibitory effects of three drugs: BIBF 1120 (nintedanib), BI 860585, and BI 836845, on signal transduction pathways.
BIBF 1120 is a triple kinase inhibitor, which blocks the receptor pathway of vascular endothelial growth factor, platelet-derived growth factor, and fibroblast growth factor [21,22]. BIBF 1120 inhibits members of the kinase family of proteins such as Src, Lck, and Lyn. In addition to the receptor-mediated signal pathways in the cells, BIBF 1120 also targets the interaction between cancer cells and the tumor microenvironment in lung cancer [23]. A phase I study suggested that BIBF 1120 has antitumor activity against many types of cancers such as colorectal cancer, renal cancer, and hepatocellular carcinoma [24]. The usefulness of BIBF 1120 as an anticancer agent against non-small cell lung cancer has been proved in preclinical and clinical phase I and II trials [25]. A phase II clinical trial on the efficacy of BIBF 1120 in metastatic soft tissue sarcomas (NCT02808247) has been recently launched.
BI 860585 is an inhibitor of mammalian target of rapamycin complex 1 (mTORC1) and mTORC2. Mammalian target of rapamycin (mTOR) is a serine/threonine kinase and a downstream effector of the PI3K/AKT pathway [26]. mTORC1 and mTORC2 are complexes formed from mTOR. mTORC1 is sensitive to rapamycin and is involved in mRNA translation, whereas mTORC2 is resistant to rapamycin and regulates actin cytoskeleton. Aberrant regulations of mTORC1 and mTORC2 have been reported in many cancers. Rapamycin and its analogues inhibit cell growth in several types of malignancies including osteosarcoma, rhabdomyosarcoma, and Ewing sarcoma [27]. The possible usefulness of mTOR inhibitors in sarcomas has also been reported [28]. An open-label phase I dose-finding study on BI 860585 is ongoing in patients with various advanced and/or metastatic solid tumors (NCT01938846). The maximum tolerated dose of BI 860585 from the study was recently reported [29].
BI 836845 is a therapeutic antibody with high and specific affinity to human insulin-like growth factor (IGF)-1 and IGF-2. BI 836845 inhibits IGF-induced activation of signal transduction [30]. IGFs control cellular proliferation and the survival and growth of organisms [31,32]. IGF-1 and IGF-2 have been noted to stimulate tumor growth in many preclinical cancer models [33]. The concentration of IGF-1 in blood is of prognostic value in cancers [34,35]. In addition, the prognostic value of IGF-1R expression in sarcomas has been reported in a meta-analysis [36]. The IGF signaling pathway is considered as a target in the treatment of sarcomas. For instance, anti-IGF-1R has been shown to be effective in the treatment of Ewing sarcoma [37]. A phase I, open-label, dose-escalation trial on the use of BI 836845 in various solid cancers (NCT01403974) was completed in December 2015.
We aimed to investigate the antitumor effects of BIBF 1120, BI 860585, and BI 836845 in 20 sarcoma cell lines. Our results will be useful in the consideration of the three inhibitors as molecular targeting drugs for the treatment of sarcoma.
Materials and methods
BIBF 1120, BI 860585, and BI 836845 were provided by Boehringer Ingelheim (Ingelheum, Germany).
Sarcoma cell lines
Twenty sarcoma cell lines with different histological backgrounds were used for the study. Table 1 shows the subtypes and sources of cell lines, as well as the culture media used in the study. The number of cells cultured per well have also been indicated. The cell lines were authenticated by examining the pattern of short tandem repeat. Mycoplasma contamination was excluded by monitoring DNA unique to mycoplasma in the culture-conditioned medium.
Growth inhibitory assay and analysis
The cells were suspended in the medium in Table 1 and plated in 96-well plates at adequate numbers for the individual cell lines and incubated overnight. The cells were then incubated with the inhibitors or vehicle for 72 hours, after which cell viability was assessed using a Cell Counting Kit 8 (Dojindo Molecular Technologies Inc, Kumamoto, Japan). Absorbance was measured at 450 nm. The software, GraphPad Prisom7 (GraphPad Software, La Jolla, CA, USA), was used to construct 4-parameter logistic curves for assessing the inhibitory effects of tyrosine kinases on cell viability. The half-maximal effective concentration (EC50) of each drug was then calculated to assess the suppressive efficacy of each drug.
Table 1. Summary of the cell lines used in the study
Sarcoma type |
Name of cell line |
Cell culture medium |
Provider of cell line |
Cells/well |
Osteosarcoma |
143B |
α |
A |
2 × 103 |
MG63 |
α |
A |
2 × 103 |
SJSA-1 |
α |
C |
4 × 103 |
U2OS |
α |
C |
5 × 103 |
HS-OS-1 |
α |
A |
2 × 103 |
HuO 9N2 |
γ |
A |
5 × 103 |
HuO 3N1 |
γ |
B |
5 × 103 |
NOS-10 |
α |
A |
1 × 103 |
HOS |
α |
A |
4 × 103 |
MNNG-HOS |
α |
C |
1 × 103 |
Synovial sarcoma |
SYO-1 |
β |
D |
5 × 103 |
1273/99 |
β |
E |
5 × 103 |
YaFuSS |
β |
F |
1 × 104 |
HS-SY-2 |
β |
A |
1.2 × 104 |
Rhabdomyosarcoma |
KYM-1 |
γ |
B |
2 × 103 |
SJCRH30 |
γ |
C |
8 × 103 |
RD |
γ |
B |
5 × 103 |
Ewing sarcoma |
A673 |
α |
C |
2 × 103 |
RD-ES |
γ |
C |
4 × 103 |
W-ES |
γ |
G |
6 × 103 |
α: Dulbecco's Modified Eagle Medium (DMEM; Sigma-Aldrich, St. Louis, MO, USA)
β: DMEM low glucose (Life Technologies, Carlsbad, CA, USA)
γ: Roswell Park Memorial Institute 1640 (Sigma-Aldrich)
All the media were supplemented with 10% fetal bovine serum, 100 U/ml penicillin G, and 100 mg/ml streptomycin (Sigma-Aldrich).
A: RIKEN BRC Cell Bank (Ibaraki, Japan)
B: Japanese Cancer Research Resources Bank (Osaka, Japan)
C: American Type Culture Collection (Rockville, MD, USA)
D: Dr. A. Kawai (National Cancer Center, Tokyo, Japan)
E: Dr. O. Larsson (Karolinska Hospital, Stockholm, Sweden)
F: Dr. J. Toguchida (Faculty of Medicine, Kyoto University, Japan)
G: Dr. Y. Fujii (Hamamatsu University, School of Medicine, Shizuoka, Japan)
Transcriptomic data analysis
Gene expression datasets of Ewing sarcoma cell lines were obtained from the public Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/) (Table 3). The data for A673, RD-ES, and W-ES cell lines have been reported previously [38,39]. Gene expression data analysis was performed using R software and other software packages from the Bioconductor Project as follows [40,41]. The DNA microarray data were normalized using MAS5.0 from the Bioconductor affy package [42] and then by the global median centering method.
Table 2. Summary of half-maximal effective concentration (EC50) values of BIBF 1120, BI 860585, and BI 836845 against sarcoma cell lines
Sarcoma subtype |
Name of cell lines |
EC50 (μM) |
BIBF 1120 |
BI 860585 |
BI 836845 |
Osteosarcoma |
14 |
2.7 |
0.11 |
>10 |
MG63 |
3.7 |
0.04 |
>10 |
SJSA-1 |
3.3 |
0.40 |
>10 |
U2OS |
2.8 |
0.36 |
>10 |
HS-OS-1 |
2.6 |
0.03 |
>10 |
HuO 9N2 |
5.7 |
0.49 |
>10 |
HuO 3N1 |
3.4 |
0.08 |
>10 |
NOS-10 |
2.3 |
0.05 |
>10 |
HOS |
3.1 |
0.39 |
>10 |
MNNG-HOS |
2.7 |
0.07 |
>10 |
Synovial sarcoma |
SYO-1 |
0.41 |
0.15 |
>10 |
1273/99 |
5.4 |
0.33 |
>10 |
YaFuSS |
0.7 |
0.13 |
8.83 |
HS-SY-2 |
0.33 |
0.04 |
6.67 |
Rhabdomyosarcoma |
KYM-1 |
3.4 |
0.07 |
>10 |
SJCRH30 |
3.8 |
0.52 |
>10 |
RD |
3.1 |
0.24 |
>10 |
Ewing sarcoma |
A673 |
3.5 |
0.18 |
>10 |
RD-ES |
6.7 |
0.08 |
0.02 |
W-ES |
5.9 |
0.06 |
0.19 |
Table 3. mRNA expression data of the Ewing sarcoma cell lines
A673 |
ArrayExpress |
E-MTAB-37 |
Affymetrix Human Genome U133 Plus 2.0 Array |
A-673_SS271873_HG-U133_Plus_2_HCHP-167936_.CEL |
RD-ES |
GEO |
GSE8596 |
Affymetrix Human Genome U133 Plus 2.0 Array |
GSM213308 |
W-ES |
GEO |
GSE8596 |
Affymetrix Human Genome U133 Plus 2.0 Array |
GSM213312 |
Results and discussion
The effects of BIBF 1120, BI 860585, and BI 836845 on the viabilities of the 20 sarcoma cell lines are illustrated in Figures 1-4. Sarcomas consist of a wide variety of histologically different mesenchymal tumors. Each sarcoma subtype has unique molecular backgrounds; therefore, therapeutic strategies are established according to the clinical and pathological features of each sarcoma [43]. Osteosarcoma, rhabdomyosarcoma, synovial sarcoma, and Ewing sarcoma are among the major sarcoma subtypes. We investigated the histological subtypes and cell lines of the sarcomas that showed favorable responses to the treatments with the inhibitors. Figures 1-4 show the logistic curves for the three drugs against the growth of each cell line. The data obtained from the curves were used to calculate the EC50 values (Table 2), which have been illustrated as a heat-map in Figure 5.
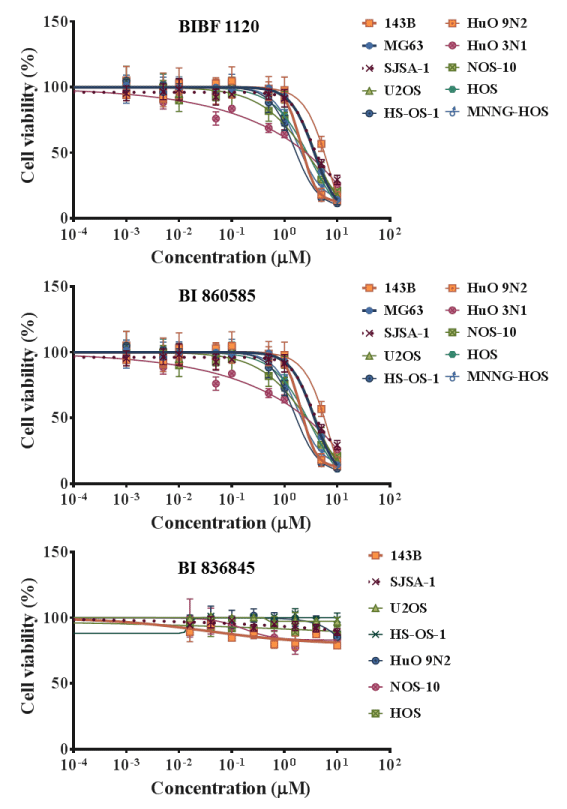
Figure 1. Dose response curves showing the inhibitory effects of BIBF 1120, BI 860585, and BI 836845 on osteosarcoma cell lines.
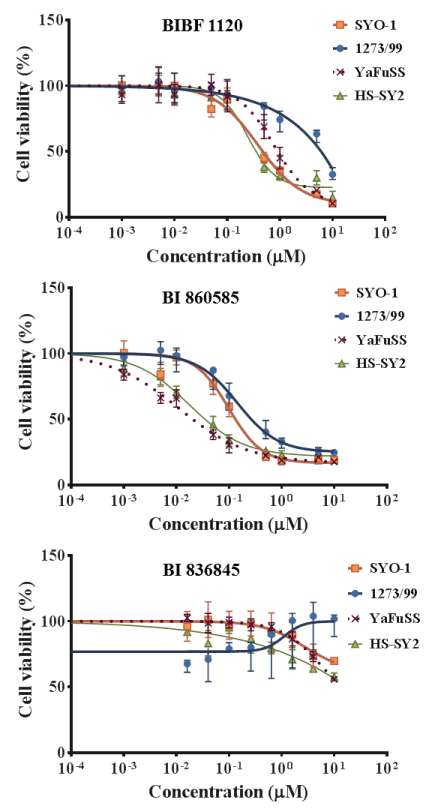
Figure 2. Dose response curves showing the inhibitory effects of BIBF 1120, BI 860585, and BI 836845 on synovial sarcoma cell lines.
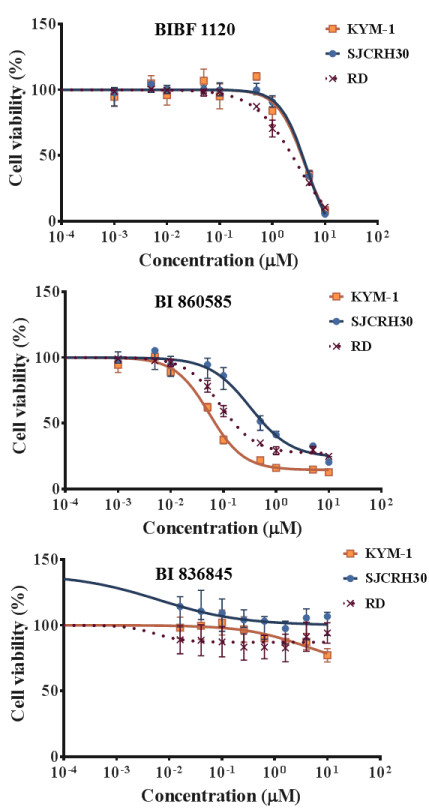
Figure 3. Dose response curves showing the inhibitory effects of BIBF 1120, BI 860585, and BI 836845 on rhabdomyosarcoma cell lines.
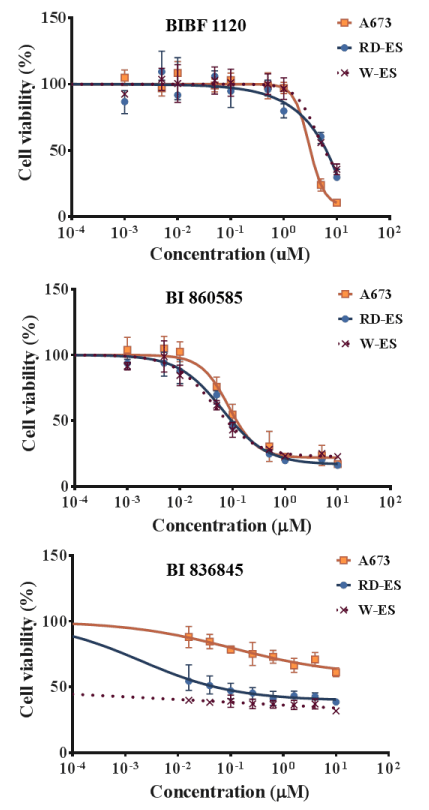
Figure 4. Dose response curves showing the inhibitory effects of BIBF 1120, BI 860585, and BI 836845 on Ewing sarcoma cell lines.
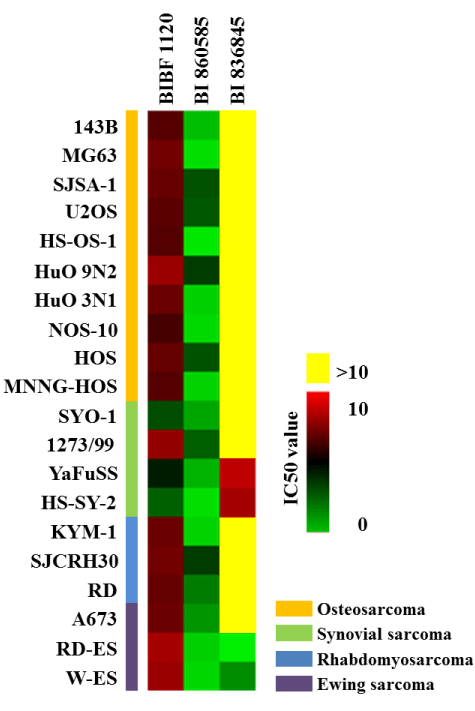
Figure 5. Half-maximal effective concentration (EC50) values of BIBF 1120, BI 860585, and BI 836845 against 20 subtypes of sarcoma cell lines. The different colors correspond to numerical data that indicate the respective EC50 values. The data have been summarized in Table 2.
BIBF 1120 showed lower EC50 values against the growth of SYO-1, YaFuSS, and HS-SY-2 cell lines than against the other sarcoma cell lines (Figure 5, Table 2). The common molecular backgrounds of SYO-1, YafuSS, and HS-SY-2 cells are therefore worth investigating in comparison with those of the other sarcoma cell lines studied. The molecular backgrounds shared among the three cell lines may include biomarkers for use in companion diagnostics. Our results show that a possible indication for BIBF 1120 is synovial sarcoma. However, sarcoma cell lines that were not used in this study are also worth investigating.
BI 860585 exhibited similar EC50 values among the 20 sarcoma cell lines (Figure 5, Table 2). Activation of the mTOR pathway and its possible utility for novel therapy have been reported in studies on osteosarcoma [44], synovial sarcoma [45], rhabdomyosarcoma [46], and Ewing sarcoma [47]. Our data may support the indication of mTOR inhibitors for those sarcomas; however, BI 860585 is worth investigating for its inhibitory effects on other sarcoma cell lines.
BI 836845 showed the lowest EC50 values against two Ewing sarcoma cell lines (RD-ES and W-ES); however, the EC50 values against the A673 cell line exceeded the calculation limit in this study (Figure 5, Table 2). The IGF-1R pathway has been considered as a target for the treatment of Ewing sarcoma [48,49]. We observed that the inhibitory effects of BI 836845 on the viability of the Ewing sarcoma cells were consistent with those stated in previous reports. To explore the possible mechanisms responsible for the different responses of the RD-ES, W-ES, and A673 cell lines to the BI 836845 treatment, we compared the expression levels of IGF-1, IGF-2, and IGF-1R in those three cell lines. Using the publically available DNA microarray data, we found that the expression level of IGF-1R was lowest in the A673 cells (Figure 6). Cao et al. have reported that the antiproliferative activity of anti-IGF-1R antibody is positively correlated with the level of IGF-1R in rhabdomyosarcoma cells [50]. The expression level of IGF-1R may therefore be a candidate predictive biomarker for the prognoses of sarcomas treated with BI 836845. However, this hypothesis should be tested using various cell lines with different IGF-1R expression levels. In addition, the cell lines should respond differently to treatment with BI 836845.
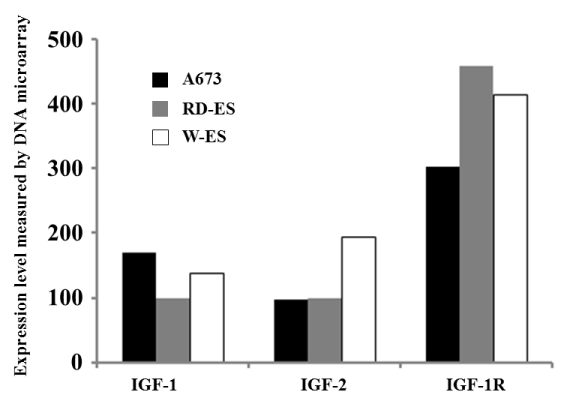
Figure 6. Expression levels of insulin-like growth factors (IGFs) in Ewing sarcoma cell lines.
Conclusions
We examined the antiproliferative effects of BIBF 1120, BI 860585, and BI 836845 against 20 sarcoma cell lines. Our results indicate that BIBF 1120 may preferentially inhibit the growths of synovial sarcoma cell lines, BI 860585 may have inhibitory effects on the viabilities of sarcoma cells with different histological features, BI 836845 may have specific antiproliferative effects in Ewing sarcoma, and IGF-1R may be a predictive biomarker for sarcomas. However, studies on the molecular backgrounds of the responses of the cell lines to the three treatments are worth conducting for the development of novel therapeutic strategies against sarcomas.
Acknowledgement
This work was supported by a research grant provided by Boehringer Ingelheim.
Conflict of interest
This study was performed in collaboration with Boehringer Ingelheim.
References
- Clark MA, Fisher C, Judson I, Thomas JM (2005) Soft-tissue sarcomas in adults. N Engl J Med 353: 701-711. [Crossref]
- Carter SK (1980) The dilemma of adjuvant chemotherapy for osteogenic sarcoma. Cancer Clin Trials 3: 29-36. [Crossref]
- Provisor AJ, Ettinger LJ, Nachman JB, Krailo MD, Makley JT, Yunis EJ, et al. (1997) Treatment of nonmetastatic osteosarcoma of the extremity with preoperative and postoperative chemotherapy: a report from the Children's Cancer Group. J Clin Oncol 15: 76-84. [Crossref]
- Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, et al. (2002) Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of ,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol 20: 776-790. [Crossref]
- Hagleitner MM, de Bont ES, Te Loo DM (2012) Survival trends and long-term toxicity in pediatric patients with osteosarcoma. Sarcoma 2012: 636405. [Crossref]
- Whelan JS, Jinks RC, McTiernan A, Sydes MR, Hook JM, et al. (2012) Survival from high-grade localised extremity osteosarcoma: combined results and prognostic factors from three European Osteosarcoma Intergroup randomised controlled trials. Ann Oncol 23: 1607-16.
- Nesbit ME Jr, Gehan EA, Burgert EO Jr, Vietti TJ, Cangir A, et al. (1990) Multimodal therapy for the management of primary, nonmetastatic Ewing's sarcoma of bone: a long-term follow-up of the First Intergroup study. J Clin Oncol 8: 1664-74. [Crossref]
- Hustu HO, Pinkel D, Pratt CB (1972) Treatment of clinically localized Ewing's sarcoma with radiotherapy and combination chemotherapy. Cancer 30: 1522-7. [Crossref]
- Jürgens H, Exner U, Gadner H, Harms D, Michaelis J, Sauer R, et al. (1988) Multidisciplinary treatment of primary Ewing's sarcoma of bone. A 6-year experience of a European Cooperative Trial. Cancer 61: 23-32. [Crossref]
- Winkler K, Beron G, Delling G, Heise U, Kabisch H, et al. (1988) Neoadjuvant chemotherapy of osteosarcoma: results of a randomized cooperative trial (COSS-82) with salvage chemotherapy based on histological tumor response. J Cli Oncol 6: 329-37. [Crossref]
- Le Deley MC, Guinebretière JM, Gentet JC, Pacquement H, Pichon F, et al. (2007) SFOP OS94: a randomised trial comparing preoperative high-dose methotrexate plus doxorubicin to high-dose methotrexate plus etoposide and ifosfamide in osteosarcoma patients. Eur J Cancer 43: 752-61. [Crossref]
- Burgert EO Jr, Nesbit ME, Garnsey LA, Gehan EA, Herrmann J, et al. (1990) Multimodal therapy for the management of nonpelvic, localized Ewing's sarcoma of bone: intergroup study IESS-II. J Clin Oncol 8: 1514-1524. [Crossref]
- Grier HE, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, et al. (2003) Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med 348: 694-701. [Crossref]
- Patel SR (2014) Fifty years of advances in sarcoma treatment: moving the needle from conventional chemotherapy to targeted therapy. Am Soc Clin Oncol Educ Book: 259-262. [Crossref]
- Shoushtari AN, Van Tine BA, Schwartz GK (2014) Novel treatment targets in sarcoma: more than just the GIST. Am Soc Clin Oncol Educ Book . [Crossref]
- Group EESNW (2012) The ESMO / European Sarcoma Network Working GroupGroup EESNW. Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 23 Suppl 7: 49-55.
- van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, et al. (2012) Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 379: 1879-1886.
- Young RJ, Woll PJ (2016) Eribulin in soft-tissue sarcoma. Lancet 387: 1594-1596. [Crossref]
- Gordon EM, Sankhala KK, Chawla N, Chawla SP (2016) Trabectedin for soft tissue sarcoma: current status and future perspectives. Adv Ther 3: 1055-71. [Crossref]
- Burgess M, Gorantla V, Weiss K, Tawbi H (2015) Immunotherapy in Sarcoma: Future Horizons. Curr Oncol Rep 17: 52. [Crossref]
- Hilberg F, Roth GJ, Krssak M, Kautschitsch S, Sommergruber W, et al. (2008) BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res 68: 4774-82. [Crossref]
- Santos ES, Gomez JE, Raez LE (2012) Targeting angiogenesis from multiple pathways simultaneously: BIBF 1120, an investigational novel triple angiokinase inhibitor. Investigational New Drugs 30: 1261-9. [Crossref]
- Epstein Shochet G, Israeli-Shani L, Koslow M, Shitrit D (2016) Nintedanib (BIBF 1120) blocks the tumor promoting signals of lung fibroblast soluble microenvironment. Lung Cancer 96: 7-14. [Crossref]
- Mross K, Stefanic M, Gmehling D, Frost A, Baas F, et al. (2010) Phase I study of the angiogenesis inhibitor BIBF 1120 in patients with advanced solid tumors. Clin Cancer Res 16: 311-9. [Crossref]
- Bronte G, Passiglia F, Galvano A, Barraco N, Listì A, et al. (2016) Nintedanib in NSCLC: evidence to date and place in therapy. Ther Adv Med Oncol 8: 188-97.
- [Crossref] Pópulo H, Lopes JM, Soares P (2012) The mTOR signalling pathway in human cancer. Int J Mol Sci 13: 1886-1918.
- Humar R, Kiefer FN, Berns H, Resink TJ, Battegay EJ (2002) Hypoxia enhances vascular cell proliferation and angiogenesis in vitro via rapamycin (mTOR)-dependent signaling. FASEB J 16: 771-80. [Crossref]
- Wan X, Helman LJ (2007) The biology behind mTOR inhibition in sarcoma. Oncologist 12: 1007-1018. [Crossref]
- F DB, JPH M, M T, S R, M D, M L, et al., editors. Phase I study of mTORC1/2 inhibitor BI 860585 as single agent or with exemestane or paclitaxel in patients with advanced solid tumors. 2016 ASCO Annual Meeting; 2016; Chicago, IL, USA: J Clin Oncol.
- Friedbichler K, Hofmann MH, Kroez M, Ostermann E, Lamche HR, et al. (2014) Pharmacodynamic and antineoplastic activity of BI 836845, a fully human IGF ligand-neutralizing antibody, and mechanistic rationale for combination with rapamycin. Mol Cancer Ther 13: 399-409. [Crossref]
- Pollak M (2012) The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer 12: 159-169. [Crossref]
- Belfiore A, Malaguarnera R (2011) Insulin receptor and cancer. Endocr Relat Cancer 18: R125-147. [Crossref]
- Yang XF, Beamer WG, Huynh H, Pollak M (1996) Reduced growth of human breast cancer xenografts in hosts homozygous for the lit mutation. Cancer Res 56: 1509-1511. [Crossref]
- Pollak M (2008) Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer 8: 915-928. [Crossref]
- Khandwala HM, McCutcheon IE, Flyvbjerg A, Friend KE (2000) The effects of insulin-like growth factors on tumorigenesis and neoplastic growth. Endocr Rev 21: 215-244. [Crossref]
- Liang J, Li B, Yuan L, Ye Z (2015) Prognostic value of IGF-1R expression in bone and soft tissue sarcomas: a meta-analysis. Onco Targets Ther 8: 1949-55. [Crossref]
- Scotlandi K, Picci P (2008) Targeting insulin-like growth factor 1 receptor in sarcomas. Curr Opin Oncol 20: 419-427. [Crossref]
- Krupp M, Itzel T, Maass T, Hildebrandt A, Galle PR, et al. (2013) CellLineNavigator: a workbench for cancer cell line analysis. Nucleic Acids Res 41: D942-8. [Crossref]
- Miyagawa Y, Okita H, Nakaijima H, Horiuchi Y, Sato B, et al. (2008) Inducible expression of chimeric EWS/ETS proteins confers Ewing's family tumor-like phenotypes to human mesenchymal progenitor cells. Mol Cell Biol 28: 2125-37. [Crossref]
- Ihaka R RG (1996) A launguage for data analysis and graphics. J Comput Graph Stat 5: 299-314.
- Reimers M, Carey VJ (2006) Bioconductor: an open source framework for bioinformatics and computational biology. Methods Enzymol 411: 119-134. [Crossref]
- Gautier L, Cope L, Bolstad BM, Irizarry RA (2004) affy--analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 20: 307-315. [Crossref]
- Fletcher CDM, Bridge JA, Hogendoorn P, Mertens F (2013) WHO Classification of Tumours of Soft Tissue and Bone.
- Ding L, Congwei L, Bei Q, Tao Y, Ruiguo W, et al. (2016) mTOR: An attractive therapeutic target for osteosarcoma? Oncotarget. [Crossref]
- Setsu N, Kohashi K, Fushimi F, Endo M, Yamamoto H, et al. (2013) Prognostic impact of the activation status of the Akt/mTOR pathway in synovial sarcoma. Cancer 119: 3504-3513. [Crossref]
- Kaylani SZ, Xu J, Srivastava RK, Kopelovich L, Pressey JG, et al. (2013) Rapamycin targeting mTOR and hedgehog signaling pathways blocks human rhabdomyosarcoma growth in xenograft murine model. Biochem Biophys Res Commun 435: 557-61. [Crossref]
- Subbiah V, Kurzrock R (2012) Ewing's sarcoma: overcoming the therapeutic plateau. Discov Med 13: 405-415. [Crossref]
- van Maldegem AM, Bovée JV, Peterse EF, Hogendoorn PC, Gelderblom H (2016) Ewing sarcoma: The clinical relevance of the insulin-like growth factor 1 and the poly-ADP-ribose-polymerase pathway. Eur J Cancer 53: 171-80. [Crossref]
- Scotlandi K, Benini S, Sarti M, Serra M, Lollini PL, et al. (1996) Insulin-like growth factor I receptor-mediated circuit in Ewing's sarcoma/peripheral neuroectodermal tumor: a possible therapeutic target. Cancer Res 56: 4570-4. [Crossref]
- Cao L, Yu Y, Darko I, Currier D, Mayeenuddin LH, et al. (2008) Addiction to elevated insulin-like growth factor I receptor and initial modulation of the AKT pathway define the responsiveness of rhabdomyosarcoma to the targeting antibody. Cancer Res 68: 8039-48. [Crossref]






