Abstract
The electrocardiographic interval between the peak and the end of the T-wave (Tpe) is believed to be an arrhythmic risk marker. However, there are also a number of reports that are inconsistent with the usefulness of Tpe for identifying abnormal repolarization. This study was designed to investigate how the Tpe prolongation is correlated to a prolonged QT interval, induced by IKr-blockers. The study included two data sets. A first group of 21 healthy subjects received 160 mg and 320 mg doses of d,l-sotalol. The second group, of 40 patients with schizophrenia, was switched to 16 mg sertindole treatment. The Fridericia corrected QT prolongations (QTcF) and the mean Tpe changes (∆Tpe) were: d,l-sotalol 160 mg: ∆QTcF=29 ms & ∆Tpe=4.7 ms, 320 mg: ∆QTcF=51 ms & ∆Tpe=6.2 ms, sertindole 16 mg: ∆QTcF=17 ms & ∆Tpe=8.5 ms. There were low correlations (r) between ∆QTcF and ∆Tpe in both d,l-sotalol groups and sertindole group.
Given the lack of linear relationship between Tpe and QT in response to potential torsadogenic drugs, this study raises doubt about the usefulness of Tpe as a biomarker for repolarization changes and torsadogenic potential in drug safety studies.
Key words
Electrocardiogram, Tpeak-Tend interval, QT interval, IKr-blocker
Introduction
Features of the morphology of the T-wave of the body surface electrocardiogram (ECG) can reflect abnormalities of ventricular repolarization. For example, the T-wave peak-to-end (Tpe), which is defined as the interval from the peak of the T-wave to the end of the T-wave, may be a relatively simple marker for observing repolarization changes and identifying drugs with torsadogenic potential.
There is some evidence suggesting that a prolonged Tpe interval represents repolarization heterogeneity and that the interval may be a marker of arrhythmogenesis [1-4]. The prolonged Tpe interval might be associated with a high risk for developing torsades de pointes (TdP) in patients with acquired and congenital long QT syndromes (LQTS). The Tpe interval can also be prolonged in patients with a history of drug-related TdP [5-11].
However, there are also a number of reports about inconsistencies of the Tpe interval as a sensitive biomarker for identifying abnormal repolarization [8,12-18]. Importantly, these reports show that the clinical usefulness of the Tpe interval for identifying patients at risk of ventricular arrhythmias is still far from being established.
The Tpe interval was proposed to quantify transmural dispersion of repolarization (TDR), based on results from the wedge preparation obtained from the free wall of the canine left ventricle [19]. The measure of dispersion was based on differences in the action potential durations (APD) of the principal layers that comprise the ventricular myocardium. It was demonstrated in this setting that the peak of the T-wave coincided with the end of epicardial repolarization, whereas the end of the T-wave coincided with the end of repolarization of the mid-mural M cells. Therefore, by testing, e.g., d-sotalol, a known QT prolonging drug, Antzelevitch et al. showed that longer APDs in the M cells with relatively shorter APDs in endocardial and epicardial layers lead to Tpe prolongation [20,21].
In the case of more complex T-waves, including negative, biphasic and triphasic ones, it was suggested that the estimation of TDR could be based on the interval from the nadir of the T-wave to the end of the T-wave instead of the interval from the peak of the T-wave to the end of the T-wave [22]. However, the representation of TDR in the ECG is a controversial subject [23,24].
The Tpe to QT interval ratio (Tpe/QT) has also been proposed to provide an estimate of dispersion of repolarization. This measurement estimates the Tpe interval relative to the total duration of activation and repolarization and since the QT interval varies with heart rate, the Tpe/QT ratio could also be less heart rate dependent than Tpe itself [25]. The Tpe/QT ratio has thus been suggested as a more sensitive index of repolarization changes and risk of arrhythmia compared to the Tpe interval alone. However, in one study of the Tpe interval we showed that Tpe was heart rate dependent but the effect of rate was only minor at near resting heart rates [16]. We have also shown that, despite QTc prolonging effects of sertindole, no increase in the duration of the Tpe interval was found [18]. In addition, we have shown that in patients with the long QT syndrome and elevated risk of TdP, it was not possible to use Tpe to distinguish symptomatic from asymptomatic patients [16]. In comparisons of ECG data from survivors and non-survivors of cardiovascular disease, Smetana et al., showed that the Tpe interval was significantly shorter in non-survivors, both uncorrected and corrected for heart rate [15]. Therefore, there are serious problems with a direct extrapolation of the findings in vitro about the Tpe interval to clinical populations. Particularly, Opthof et al. argued that there are influential biological differences between in vitro preparations and the in vivo heart, such as autonomic, humoral, and hemodynamic factors [26].
In addition, there is a small body of data dealing with the functional role of M cells in the human heart. Some data has failed to show the role of M cells in effecting Tpe changes [27,28]. According to the studies by Opthof et al., the Tpe interval is unlikely to be correlated with TDR in vivo [26,29]. These studies conclude that, in general, epicardial repolarization occurs later than endocardial repolarization in the free wall of the left ventricle. Hence, the functional and clinical importance of M cells for Tpe changes in vivo is not clear.
It thus remains controversial what the Tpe interval actually represents. However, it is important to quantify the extent to which the Tpe interval is correlated with the whole heart repolarization time, represented by the QT interval.
Aim of the Study
The present study was designed to examine the association between the Tpe interval and QT prolongation induced by two torsadogenic drugs: sertindole and d,l-sotalol, both IKr-blockers, capable of inducing QT prolongation and TdP [30,31].
Methods
Study Population and Design
The present study included data from two different IKr-blockers. The first group of 39 healthy subjects received 0, 160 mg and 320 mg doses of d,l-sotalol on three consecutive days. All subjects were males, between 18 and 45 years of age. Their healthy status was confirmed by history, physical examination, normal blood pressure and no use of concomitant medication. The second group of 37 patients carried a WHO ICD-10 diagnosis of schizophrenia. This group was switched to 16 mg sertindole treatment. The study was approved by the Scientific Ethical Committee of Northern Jutland. None of the subjects had a history of cardiac diseases and all had normal baseline ECG. In some cases the V5 on ECG data could not be analysed based on signal noise. Further demographic information on subjects receiving d,l-sotalol and sertindole can be found in Sarapa et al. , Graff et al. and Nielsen et al. [32-34].
All subjects gave written informed consent to the study protocol approved by an independent Institutional Review Board. All subjects gave written informed consent to the study protocol approved by an independent Institutional Review Board. All subjects gave written informed consent to the study protocol approved by an independent Institutional Review Board. All subjects gave written informed consent to the study protocol approved by an independent Institutional Review Board. All subjects gave written informed consent to the study protocol approved by an independent Institutional Review Board. All subjects gave written informed consent to the study protocol approved by an independent Institutional Review Board. All subjects gave written informed consent to the study protocol approved by an independent Institutional Review Board. All subjects gave written informed consent to the study protocol approved by an independent Institutional Review Board.
Written informed consent was obtained from all subjects in the first group for the study protocol. However, in the second group obtaining informed consent was not required because the study was non-interventional and ECG was indicated for therapeutic monitoring.
ECG Acquisition
For d,l-sotalol, ECG segments of 10 s duration were obtained from 12-lead digital Holter recordings (H12Recorder, Mortara Instrument, Milwaukee, WI). ECG recordings at the time of maximum plasma concentrations, 3.5 h post-dose were used, both for 160 mg and 320 mg d,l-sotalol. The corresponding study time was used on the baseline day. Each ECG extracted from Holter was resampled from 180 Hz to 500 Hz as previously described [33].
For sertindole, digital ECGs were recorded with GE MAC5000 and GE Cardiosoft (GE Healthcare, Milwaukee, WI). At baseline five consecutive 12-lead digital ECGs of 10 sec duration were recorded from each schizophrenia patient at a sample rate of 500 Hz and transferred to a MUSE database (GE Healthcare, Milwaukee, WI). ECGs were recorded again when patients reached steady-state concentration of sertindole 16 mg, after a minimum of 3 weeks as recommended in the Summary of Product Characteristics of Serdolect [34].
ECG Analysis
From each 10 s ECG recording a median beat was formed in the recorded leads using MUSE/Interval Editor software (GE Healthcare, Milwaukee, WI). Lead V5 was used to measure the Tpe interval because V5 is the lateral precordial lead which is considered to best reflect the electrical phenomena in the left ventricle during IKr-blocker-induced repolarization disorders. Based on the early studies, lead V5 was targeted to measure the Tpeak-Tend interval to predict TdP [5]. Since analyses were performed on a median beat, ECGs were not filtered to measure Tpeak location.
QT intervals were measured automatically (12SL, GE Healthcare, Milwaukee, WI). QT intervals were corrected for heart rate with Fridericia’s equation: QTcF=QT/RR1/3.
The peak of the T-wave was defined as the point of highest amplitude of the T-wave. In cases of notched or bifid T-waves the interval from the nadir, between the peaks of the T-wave, to the end of the T-wave was used [20-22].
Statistical Analysis
Data were analyzed using Matlab R2012b (Mathworks Inc, Natick, MA). Results are presented as means and standard deviations. Pearson’s correlation coefficients were used for investigating linear relationships. All findings were compared by Student’s t-test for paired data. A p-value <0.05 was considered statistically significant.
Results
ECG characteristics for the sertindole and d,l-sotalol groups are outlined in table 1. The mean Tpe did change significantly from baseline with sertindole and for d,l-sotalol 320 mg, but not significantly for 160 mg. Also, the QT and QTcF intervals increased significantly following both d,l-sotalol and sertindole administration (Figure 1).
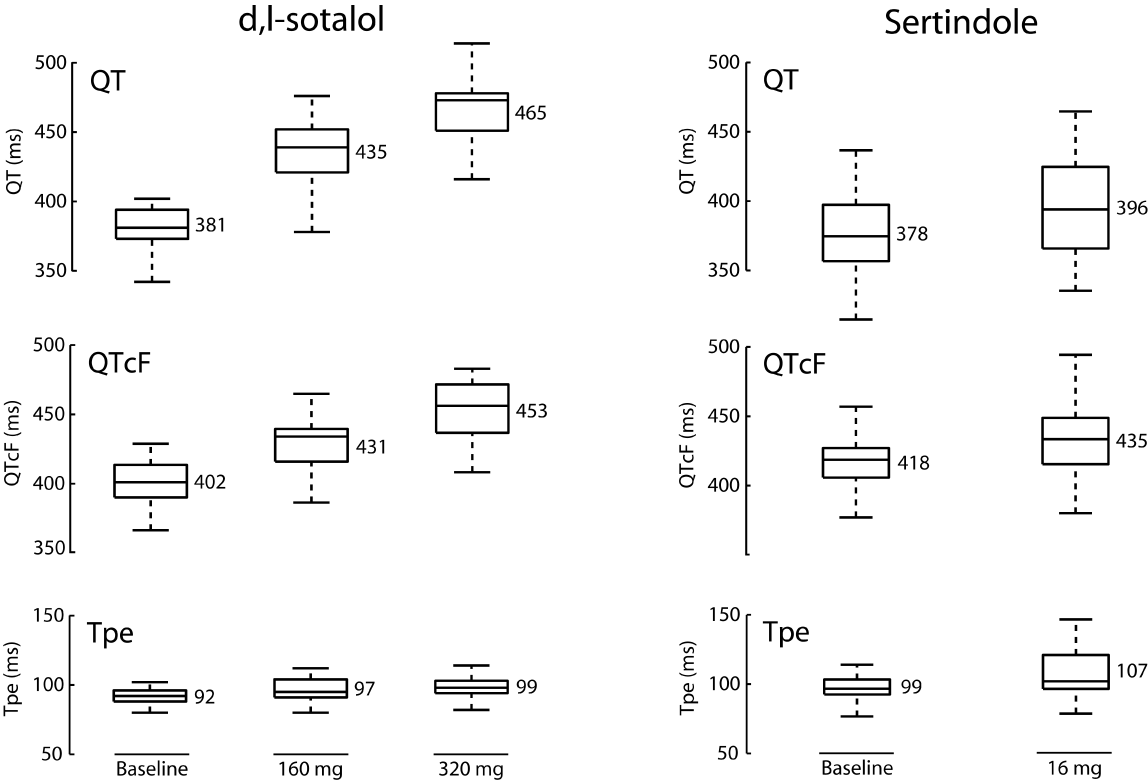
Figure 1: QT, QTcF and Tpe for d,l-sotalol (left column) and sertindole (right column) Boxplots: median, interquartile range and full range. Mean values are given for each measurement.
Table 1: ECG characteristics for sertindole and sotalol.
|
Sertindole |
Sotalol |
Baseline |
treatment |
p-value* |
baseline |
160mg |
p-value |
320mg |
p-value |
QT (ms) |
378 ± 56 |
396 ± 71 |
<0.05 |
381 ± 35 |
435 ± 51 |
<0.001 |
465 ± 50 |
<0.001 |
Tpe (ms) |
99 ± 24 |
107 ± 34 |
<0.05 |
92 ± 16 |
97 ± 19 |
0.09 |
98.5 ± 18 |
<0.05 |
QTcF (ms) |
418 ± 42 |
435 ± 48 |
<0.05 |
402 ± 31 |
431 ± 39 |
<0.001 |
453 ± 43 |
<0.001 |
*p-values for sertindole and d,l-sotalol versus baseline.
A scatter plot for Tpe versus QTcF, for all data, is shown in figure 2. Correlation coefficients for the linear relationships are given in table 2. The largest correlation coefficient (r) was r=0.45, p=0.005 for sertindole, whereas the correlation coefficients for Tpe on QTcF for sotalol were small and not significant (160 mg: r=0.15, p=0.54 and 320 mg: r=0.15, p=0.54). Despite the statistically significant correlation for sertindole between QTcF and Tpe, the relationship explains only a small percentage (20%) of the variation of Tpe with QTcF.
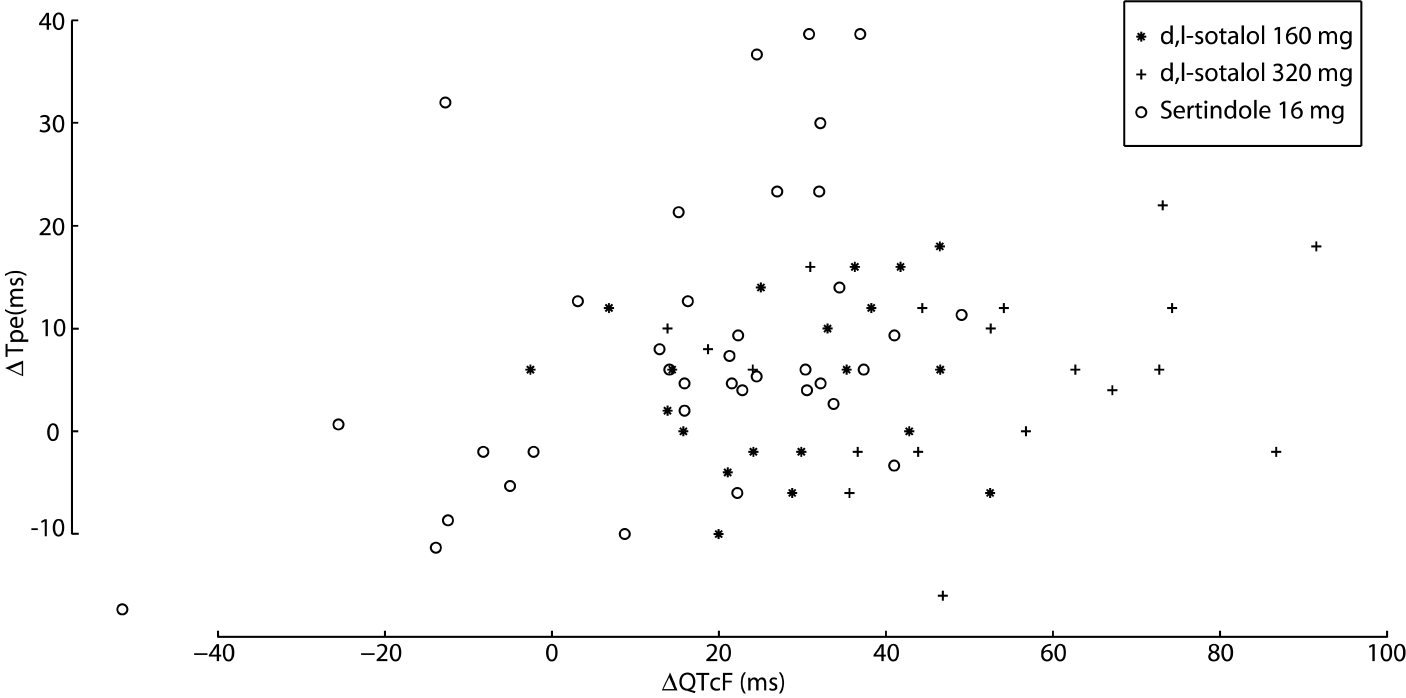
Figure 2: Scatter plot for changes in QTcF and Tpe from baseline for sotalol 160 mg (*), sotalol 320 mg (+) and sertindole (o)
Table 2: Pearson’s correlation coefficients between prolongation of QTcF and Tpe intervals.
∆QTcF & ∆Tpe |
Pearson Correlation Coefficient (r) |
p-value |
d,l-sotalol (320mg) |
0.15 |
0.52 |
d,l-sotalol (160mg) |
0.15 |
0.54 |
Sertindole (16 mg) |
0.45 |
0.005 |
Discussion
We found that, despite marked QT and QTcF prolongation, induced by potential torsadogenic IKr-blockers d,l-sotalol and sertindole, little linear association was observed between the Tpe interval and the QTcF interval. Tpe changes with a lower significant value (see P-value in Table 1). Figure 2 shows a high variance of Tpe relative to the drug-induced changes and, compared with QTcF, Tpe therefore is not a robust predictor of potential torsadogenicity prolonging repolarization.
Our results are not in accordance with a number of previous studies [1-11]. On the basis of prolonged Tpe intervals, those studies concluded that the Tpe interval can be an important marker of arrhythmia susceptibility. However, there is also a number of studies that support our findings whether prolongation of the Tpe interval can be used to predict the risk of arrhythmia [12-18]. In one case, Tpe was even found to be statistically significantly shorter in non-surviving cardiovascular patients compared to survivors [15].
Our studies on T-wave morphology have shown that, compared to the QT interval, T-wave morphology is a more sensitive marker for assessing repolarization changes and arrhythmic potential with IKr-blockers [33-36]. This is not surprising since the electrophysiological effects that occur with torsadogenic drugs are expected to have a larger effect on T-wave changes and a stronger association with TdP compared to the QT interval [37]. In addition, T-wave shape changes are not captured by the Tpe interval which measures only the duration of the poorly defined final part of repolarization. Interestingly, our recent work has proposed the minimal T-wave representation by making T-wave independent of QT duration and thus much less dependent on heart rate. The results of analyzing the minimal T-wave shape showed that it could identify normal from abnormal repolarization significantly better compared to heart rate corrected QT interval (QTc) and most of the prolongation occurred in the ascending segment of the T-wave whereas T-peak to T-end was less sensitive relatively after d,l-sotalol [38]. Also, studies of the effects of some IKr blockers on multiple sub-intervals of the QT interval showed that hERG potassium channel block equally affects the whole T-wave duration, early and late repolarization (Tj to Tend and Tpe intervals) whereas additional inward current block (calcium or late sodium) preferentially shortens early repolarization [39,40]. In addition, Johannesen et al. showed that QTc (QTc prolongation in 20 of 26 drugs, P=0.014) is a more sensitive biomarker of hERG potassium channel block compared with Tpe (Tpe prolongation in 18 of 26 drugs, P=0.051) [40]. The measurement of Tpe alone can therefore, not be expected to provide adequate information about the complex repolarization changes that occur with torsadogenic drugs.
There are two important sources of error in the Tpe interval. First, the end of the T-wave can be difficult to determine, since it may not reach the baseline and may be followed by a superimposed U-wave or P-wave. This is the same problem as occurs when determining the QT interval. Secondly, IKr-blockers may produce considerable T-wave changes particularly flatness, notches and asymmetry. The drug-induced shape changes add to the difficulty of measuring the time of occurrence of the peak of the T-wave, which thus has a great influence on the assessment of changes of the Tpe interval. Due to the short duration of the Tpe interval, those measurement errors will have a relatively larger impact on absolute values of the Tpe interval compared to the QT interval. This is an undesirable property of the Tpe interval as a biomarker for repolarization changes. Finally, the absolute values for the Tpe interval is heavily influenced by the method used to measure the duration. For example, the tangent method will tend to measure QT and Tpe intervals which are shorter compared to the method used in this study which estimate the end of the T-wave from superimposition of recorded leads. The discrepancy between measuring methods explains why studies that do not use the same method for estimating the end of the T-wave, can report very different QT and Tpe intervals, even on the same data. The discrepancy in measuring technique does not explain however, the lack of effect on the Tpe interval in this study, despite marked QT interval changes.
Based on Xia et al. it is not useful to average Tpe as index of TDR, among several leads because TDR might vary in different regions of the ventricular myocardium under different pathophysiologic conditions [41]. Therefore, it is advisable to measure Tpe independently in each of the precordial leads. Also, early studies used the V5 lead reflecting electrical phenomena in left ventricle to show that the Tpe interval of the T-wave loop might be a useful predictor of TdP in long QT syndrome (LQTS) patients whose IKr current is (partially) blocked. Consequently, in this study lead V5 was targeted to measure the Tpe interval, because it could be considered to best reflect the electrical phenomena in the left ventricle during IKr-blocker-induced repolarization disorders.
One limitation of the study is that different devices were used in the two groups for ECG recording. Also, this study is based on a retrospective analysis and that the conclusion should be interpreted with appropriate levels of caution.
In conclusion, we have shown much lower effect sizes for drug-induced changes of Tpe than for QTcF, which implies that QTcF is a more robust biomarker for repolarization changes in drug safety studies.
Compliance with Ethical Standards
Funding
This study was partially supported by the Region Nordjyllands Sundhedsvidenskaelige Forskningsfond (Health Research Fund of Central Denmark Region).
Conflict of interest
Regarding this work the authors do not report any conflict of interest.
Ethical approval
References
- Lubinski A, Kornacewicz-Jach Z, Wnuk-Wojnar AM, Adamus J, Kempa M, Królak T, et al. (2000) The terminal portion of T wave: A new electrocardiographic marker of risk of ventricular arrhythmias. Pacing Clin Electrophysiol. 23: 1957-1959. [Crossref]
- Watanabe N1, Kobayashi Y, Tanno K, Miyoshi F, Asano T, et al. (2004) Transmural dispersion of repolarization and ventricular tachyarrhythmias. J Electrocardiol. 37: 191-200. [Crossref]
- Panikkath R1, Reinier K, Uy-Evanado A, Teodorescu C, Hattenhauer J, et al. (2011) Prolonged Tpeak to Tend interval on the resting electrocardiogram is associated with increased risk of sudden cardiac death. Circ Arrhythm Electrophysiol. 4: 441-447. [Crossref]
- Haarmark C1, Hansen PR, Vedel-Larsen E, Pedersen SH, Graff C, et al. (2009) The prognostic value of the Tpeak-Tend interval in patients undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. J Electrocardiol. 42: 555-560. [Crossref]
- Yamaguchi M, Shimizu M, Ino H, Terai H, Uchiyama K, et al. (2003) T wave peak-to-end interval and QT dispersion in acquired long QT syndrome: a new index for arrhythmogenicity. Clin Sci (Lond) 105: 671-676. [Crossref]
- Lubinski A, Lewicka-Nowak E, Baczynska AM, Romanowska I, Swiatecka G (1998) New insight into repolarization abnormalities in patients with congenital long QT syndrome: the increased transmural dispersion of repolarization. Pacing Clin Electrophysiol. 21: 172-175. [Crossref]
- Takenaka K, Ai T, Shimizu W, Kobori A, Ninomiya T, et al. (2003) Exercise stress test amplifies genotype-phenotype correlation in the LQT1 and LQT2 forms of the long-QT syndrome. Circulation. 107: 838-844. [Crossref]
- Viitasalo M1, Oikarinen L, Swan H, Väänänen H, Glatter K, et al. (2002) Ambulatory electrocardiographic evidence of transmural dispersion of repolarization in patients with long-QT syndrome types 1 and 2. Circulation. 106: 2473-2478. [Crossref]
- Shimizu W1, Tanabe Y, Aiba T, Inagaki M, Kurita T, et al. (2002) Differential effects of beta-blockade on dispersion of repolarization in the absence and presence of sympathetic stimulation between the LQT1 and LQT2 forms of congenital long QT syndrome. J Am Coll Cardiol. 39: 1984-1991. [Crossref]
- Couderc JP, Kaab S, Hinterseer M, S. McNitt, X. Xia, et al. (2007) Investigating the role of ventricular repolarization morphology in surface ECGs for identifying patients with a history of drug-induced arrhythmias. Comput Cardiol. 34: 337-340.
- Couderc JP, Kaab S, Hinterseer M, McNitt S, Xia X, et al. (2009) Baseline values and sotalol-induced changes of ventricular repolarization duration, heterogeneity, and instability in patients with a history of drug-induced torsades de pointes. J Clin Pharmacol. 49: 6-16. [Crossref]
- Wolk R, Mazurek T, Lusawa T, Wasek W, Rezler J (2001) Left ventricular hypertrophy increases transepicardial dispersion of repolarisation in hypertensive patients: a differential effect on QTpeak and QTend dispersion. Eur J Clin Invest. 31: 563-569. [Crossref]
- Taggart P, Sutton PM, Opthof T, Coronel R, Trimlett R, et al. (2001) Transmural repolarisation in the left ventricle in humans during normoxia and ischaemia. Cardiovasc Res 50: 454-462. [Crossref]
- Smetana P, Pueyo E, Hnatkova K, Batchvarov V, Camm AJ, et al. (2003) Effect of amiodarone on the descending limb of the T wave. Am J Cardiol 92: 742-746. [Crossref]
- Smetana P1, Schmidt A, Zabel M, Hnatkova K, Franz M, et al. (2011) Assessment of repolarization heterogeneity for prediction of mortality in cardiovascular disease: peak to the end of the T wave interval and nondipolar repolarization components. J Electrocardiol. 44: 301-308. [Crossref]
- Kanters JK, Haarmark C, Vedel-Larsen E, Andersen MP, Graff C, et al. (2008) T(peak)T(end) interval in long QT syndrome. J Electrocardiol 41: 603-608. [Crossref]
- Porthan K, Marjamaa A, Viitasalo M, Väänänen H, Jula A, et al. (2010) Relationship of common candidate gene variants to electrocardiographic T-wave peak to T-wave end interval and T-wave morphology parameters. Heart Rhythm. 7: 898-903. [Crossref]
- Nielsen J, Andersen MP, Graff C, Kanters JK, Hardahl T, et al. (2010) The effect of sertindole on QTD and TPTE. Acta Psychiatr Scand 121: 385-388. [Crossref]
- Yan GX, Shimizu W, Antzelevitch C (1998) Characteristics and distribution of M cells in arterially perfused canine left ventricular wedge preparations. Circulation 98: 1921-1927. [Crossref]
- Shimizu W, Antzelevitch C (1998) Cellular basis for the ECG features of the LQT1 form of the long-QT syndrome. Effects of beta-adrenergic agonists and antagonists and sodium channel blockers on transmural dispersion of repolarization and Torsade des Pointes. Circulation. 98: 2314-2322. [Crossref]
- Shimizu W, Antzelevitch C (1997) Sodium channel block with mexilitine is effective in reducing dispersion of repolarization and preventing Torsade de Pointes in LQT2 and LQT3 models of the long-QT syndrome. Circulation. 96: 2038-2047. [Crossref]
- Emori T, Antzelevitch C (2001) Cellular basis for complex T waves and arrhythmic activity following combined I(Kr) and I(Ks) block. J Cardiovasc Electrophysiol. 12: 1369-1378. [Crossref]
- Haraguchi Y, Yoshinaga M, Sarantuya J, Shimago A, Nishi J, et al. (2005) Interval representative of transmural dispersion of repolarization in children and young adolescents with congenital long QT syndrome. Circ J 69: 78-82. [Crossref]
- Inoue M, Shimizu M, Ino H, Yamaguchi M, Terai H, et al. (2003) Q-T peak dispersion in congenital long QT syndrome: possible marker of mutation of HERG. Circ J 67: 495-498. [Crossref]
- Gupta P, Patel C, Patel H, Narayanaswamy S, Malhotra B, et al. (2008) T(p-e)/QT ratio as an index of arrhythmogenesis. J Electrocardiol 41: 567-574. [Crossref]
- Opthof T, Coronel R, Janse MJ (2009) Is there a significant transmural gradient in repolarization time in the intact heart? Repolarization gradients in the intact heart. Circ Arrhythm Electrophysiol. 2: 89-96. [Crossref]
- Janse MJ, Coronel R, Opthof T (2011) Counterpoint: M cells do not have a functional role in the ventricular myocardium of the intact heart. Heart Rhythm 8: 934-937. [Crossref]
- Wilson LD, Jennings MM, Rosenbaum DS (2011) Point: M cells are present in the ventricular myocardium. Heart Rhythm 8: 930-933. [Crossref]
- Opthof T, Coronel R, Wilms-Schopman FJ, Plotnikov AN, Shlapakova IN, et al. (2007) Dispersion of repolarization in canine ventricle and the electrocardiographic T wave: Tp-e interval does not reflect transmural dispersion. Heart Rhythm. 4: 344-348. [Crossref]
- Moore N (2002) Higher cardiovascular mortality with sertindole in ADROIT: a signal not confirmed. Int J Psychiatry Clin Pract 6: 3-9. [Crossref]
- Kühlkamp V, Mermi J, Mewis C, Seipel L (1997) Efficacy and proarrhythmia with the use of d,l-sotalol for sustained ventricular tachyarrhythmias. J Cardiovasc Pharmacol 29: 373-381. [Crossref]
- Sarapa N, Morganroth J, Couderc JP, Francom SF, Darpo B, et al. (2004) Electrocardiographic identification of drug-induced QT prolongation: assessment by different recording methods. Ann Noninvasive Electrocardiol. 9: 48-57. [Crossref]
- Graff C, Andersen MP, Xue JQ, Hardahl TB, Kanters JK, et al. (2009) Identifying drug-induced repolarization abnormalities from distinct ECG patterns in congenital long QT syndrome: a study of sotalol effects on T-wave morphology. Drug Saf. 32: 599-611. [Crossref]
- Nielsen J, Graff C, Hardahl T, Andersen MP, Kristoffersen J, et al. (2009) Sertindole causes distinct electrocardiographic T-wave morphology changes. Eur Neuropsychopharmacol 19: 702-707. [Crossref]
- Shakibfar S, Graff C, Ehlers LH, Toft E, Kanters JK, et al. (2012) Assessing common classification methods for the identification of abnormal repolarization using indicators of T-wave morphology and QT interval. Comput Biol Med. 42: 485-491. [Crossref]
- Graff C1, Matz J, Christensen EB, Andersen MP, Kanters JK, et al. (2009) Quantitative analysis of T-wave morphology increases confidence in drug-induced cardiac repolarization abnormalities: evidence from the investigational I-Kr inhibitor Lu 35-138. J Clinical Pharmacol. 49: 1331-1342. [Crossref]
- Shah RR, Hondeghem LM (2005) Refining detection of drug-induced proarrhythmia: QT interval and TRIaD. Heart Rhythm 2: 758-772. [Crossref]
- Shakibfar S1, Graff C2, Kanters JK3,4,5, et al. (2016) Minimal T-wave representation and its use in the assessment of drug arrhythmogenicity. Ann Noninvasive Electrocardiol. [Crossref]
- Johannesen L, Vicente J, Mason JW, Sanabria C, Waite-Labott K, et al. (2014) Differentiating drug-induced multichannel block on the electrocardiogram: randomized study of dofetilide, quinidine, ranolazine, and verapamil. Clin Pharmacol Ther. 96: 549-558. [Crossref]
- Johannesen L, Vicente J, Gray RA, Galeotti L, Loring Z, et al. (2014) Improving the assessment of heart toxicity for all new drugs through translational regulatory science. Clin Pharmacol Ther. 95: 501-508. [Crossref]
- Xia Y, Liang Y, Kongstad O, Liao Q, Holm M, et al. (2005) In vivo validation of the coincidence of the peak and end of the T wave with full repolarization of the epicardium and endocardium in swine. Heart Rhythm 2: 162-169. [Crossref]
Written informed consent was obtained from all subjects in the first group for the study protocol and was approved by the Scientific Ethical Committee of Northern Jutland. However, in the second group obtaining informed consent was not required because the study was non-interventional and ECG was indicated for therapeutic monitoring. Further demographic information on subjects receiving d,l-sotalol and sertindole can be found in Sarapa et al., Graff et al. and Nielsen et al. [32-34].
All subjects gave written informed consent to the study protocol approved by an independent Institutional Review Board. All subjects gave written informed consent to the study protocol approved by an independent Institutional Review Board. All subjects gave written informed consent to the study protocol approved by an independent Institutional Review Board. All subjects gave written informed consent to the study protocol approved by an independent Institutional Review Board.
All subjects gave written informed consent to the study protocol approved by an independent Institutional Review Board. All subjects gave written informed consent to the study protocol approved by an independent Institutional Review Board. All subjects gave written informed consent to the study protocol approved by an independent Institutional Review Board. All subjects gave written informed consent to the study protocol approved by an independent Institutional Review Board.


