Abstract
Bone marrow mesenchymal stem cells MSCs) secrete paracrine factors that may exert healing effect on the wounded tissues. This study was done to determinate the effect of MSCs or MSC-Conditioned Media MSC-CM) on the wound healing in intact mice, and under hyperglycemic condition. We established a burned wound healing mice diabetes mellitus DM) model and cultured primary bone marrow MCSs. A MSCs suspension or MSC-CM was injected into wound bed. Phenotype of MSCs, multiple differentiating capacities, and wound closure was determined. Secretion of cytokines or growth factors into culture media was determined by ELISA. MSCs and MSC-CM caused a rapid wound closure. MSCs secreted into the media a wide range of paracrine factors. These data demonstrate that MSCs promote wound healing in mice with or without STZ-induce diabetes mellitus through secretion of paracrine factors.
Key words
into wound bed injection, mesenchymal stem cell, conditioned media, diabetes mellitus
Abbreviations
B6: C57Bl6 mice; DM: Diabetes Mellitus; DFU: Diabetic Foot Ulcer; GM-CSF: Granulocyte/Macrophage-Colony stimulating factor; IL-1β: Interleukin 1 beta; IL-2: Interleukin 2; IL-4: Interleukin 4; IL-6: Interleukin 6; IL-10: Interleukin 10; IFNγ: Interferon gamma; MSCs: Mesenchymal Stem Cells; MSC-CM: Mesenchymal Stem Cells-Conditioned Media; NO: Nitrite Oxide; TNFα: Tumor Necrosis Factor alpha; VEGF: Vascular endothelial growth factor
Introduction
Diabetic foot ulcers DFUs) are one of the major complications of diabetes mellitus worldwide [1]. Approximately 25% of these patients will have progressive disease that eventually leads to amputation [2-4]. Wound healing is a coordinated process comprising an inflammatory reaction, angiogenesis and formation of extracellular matrix accompanied by scar tissue remodeling. Cellular participants as well as multiple growth factors and cytokines released by the cells at the wound site regulate these processes and finally result in wound closure. Deregulated healing processes may delay repair and may eventually lead to chronic wounds, such as those observed in diabetes mellitus [5-7]. Mesenchymal stem cells MSCs) therapy has emerged as a novel and promising candidate approach for the treatment of DFUs, probably by be as progenitors of all connective tissue cells, decrease apoptosis, cell replacement and modulation on inflammation and immune response. MSCs have been shown to mobilize and home ischemic and wounded tissues where they secrete chemokines and growth factors to promote angiogenesis and extracellular matrix remodeling. Recently, MSCs transplantation has demonstrated significant wound healing in patients with DFUs [8-20]. In the present study, we established a delayed wound healing model in diabetic mice and evaluated the impact of MSCs engraftment, and injection of MSC-Conditioned Media on delayed wound healing.
Materials and methods
General procedures
All experimental protocols were approved by the Animal Care and Use Committee of the Institute of Clinical and Experimental Lymphology, Novosibirsk, Russia, in accordance with guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The protocol of anesthesia, burn induction, post-burn care and sacrifice were identical for all animals. The animals were sacrificed under deep ether general anesthesia. Animal were housed under a 12-hour light/dark cycle with free access to food and water. A total of 48 male C57Bl6 B6) mice weighing 20-25 g, were housed 5 per cage in room maintained at 22 ± 0.5°C and relative humidity of 65-70%. The animals were allowed to adapt to the laboratory for at least 2-hour before testing and were only used once. Experiments were performed during the light phase of the cycle 10:00-17:00).
Isolation of bone marrow derived mesenchymal stem cells and culture conditions
Whole bone marrow was isolated from both the femurs of intact B6 mice. Bone marrow was aspirated with a syringe with a 26-gauge needle, containing 1 mL heparin, and disaggregated into a single-cell suspension. Mononuclear cells were separated by density-gradient centrifugation over Ficoll-Paque Sigma). The cells were seeded onto culture T-25 flask at 1 × 105 cells/cm2 with 37°C/5% CO2 in culture medium containing Dulbecco’s modified Eagle’s medium DMEM; Hyclone) supplemented with 10% heat-inactivated fetal caw serum FCS, Hyclone), 80 μg/mL gentamycin Gibco). Three days later, non-adherent cells were removed by changing the medium. After 14 days in culture, adherent cells were passaged with 0.05% trypsin-EDTA Gibco). Mesenchymal stem cells-Conditioned Media MSC-CM) was prepared by plating 2 × 106 MSCs in 10 mL DMEM supplemented with 10% FCS, 80 μg/mL on T-25 flask for 48-hours. Following culture, the media was filtered through a 0.22mm membrane.
Characterization of MSCs
MCSs of passage 3 incubated with monoclonal PE-conjugated antibodies for CD34 and CD73, with FITC-conjugated antibodies for CD90, and CD45, and with APC-conjugated antibodies for CD105 BD) in the dark for 30 min at 40C. The cells were subsequently washed three times with phosphate buffer solution PBS), resuspended in 500 μL FACS buffer 0.05% NaN3 + 5% FCS in PBS) and analyzed on FACSCanto II BD) using FACSDiva BD). Adipocytes differentiation was induced in DMEM culture medium containing 1-methyl-3-isobutylxanthine, 10-9 M dexamethasone, 5 mM insulin and 5 mM indomethacin. Osteogenic differentiation was induced in DMEM culture medium containing 50 μg/mL ascorbic acid, 10 mM β-glycerophosphate and 10-9 M dexamethasone. The medium was replaced every 2-3 days for 14 days. Matured adipocytes that formed lipid vacuoles were stained with oil red O Sigma) staining. Calcium formation of cells with a rounder shape was demonstrated by staining with alizarin red S Sigma), a marker for the osteocytes phenotype.
Streptozotocin-induced diabetes mellitus
Diabetes mellitus was induced in 24 B6 male mice. B6 mice were starved for at least 12-hour before a single intraperitoneal injection of streptozotocin STZ, Sigma) dissolved in sodium citrate buffer 0.1 mM, PH 4.4) at a dose of 60 mg/kg. At day 0 and during 28 days following after STZ injection, blood samples were obtained from the tail vein, and the blood glucose levels were measured by glucometer AccuCheck Advantage and AccuCheck Comfort Strips, Roche; Branchburg, NJ). STZ-treated B6 mice with blood glucose levels above 15 mM/L were considered diabetic and were used in this study.
Wound creation and treatment
The hair was clipped from the back of each animal with standard animal clippers. 24 B6 mice with STZ-diabetes and 24 B6 mice without STZ-diabetes were anesthetized and burns were induced on the thigh using 1 × 2 cm piece of hot metal, producing a burn of up to 10% of the total body surface and extending to all layers of skin but not involving the muscular tissue. At day 0 after wounding, 1 × 106 MSCs in 200 μL PBS MSCs wounds; n=8), 200 μL MSC-CM MSC-CM wounds; n=8), and 200 μL PBS PBS wounds; n=8) were inoculated into the wound bed. The wounds were observed daily. Estimates of wound area were calculated from the product of two mutually perpendicular perimeters. Wound area was measured using a caliper. Wound healing was quantitatively measured and calculated by the remaining wound area.
Histopathological examination
Skin wound sites on day 30 after injury, excised from the mice, were fixed in 4% formalin for 4-hour at room temperature prior to embedding in paraffin. 5 μm thickness paraffin sections were dewaxed, rehydrated, washed and stained with hematoxylin-eosin H&E). At least three skin sections per mouse were analyzed.
ELISA for MSCs culture medium
Supernatants were collected from MSCs. The concentration of IL-1β, IL-2, IL-4, IL-6, IL-10, TNF-α, IFN-γ, VEGF, and GM-CSF were measured using sandwich enzyme-linked immunosorbent assay ELISA) kits eBioscience, Austria), according to the manufacturer’s instructions.
Nitrite production
Nitrite was measured as an indicator of nitrite oxide NO) production in supernatants from MSCs. NO inhibition assay was conducted using Griess reagent kit for nitrite determination Molecular Probes). The amount of NO was calculated using a sodium nitrite standard curve.
Statistical analysis
Data were analyzed by the Statistica 6.0 for Windows. All data obtained are presented as the mean ± SEM standard error of the mean). The differences between mean values were tested for significance using the nonparametric Mann-Whitney U test.
Results
MSCs culture and characterization
MSCs were successfully isolated from non-diabetic Bl6 mice bone marrow. Cells were cultured to passage three and used for cellular therapy. The cells exhibited heterogeneous morphology on day 3 and produced an adherent layer on day 14. MSCs on tissue culture plastic demonstrated spindle-shaped morphology on becoming confluent Figure 1a). To identify MSCs characteristics, phenotypes and multiple differentiating capacities of cultured BM-derived adherent cells at passage three were analyzed, respectively.
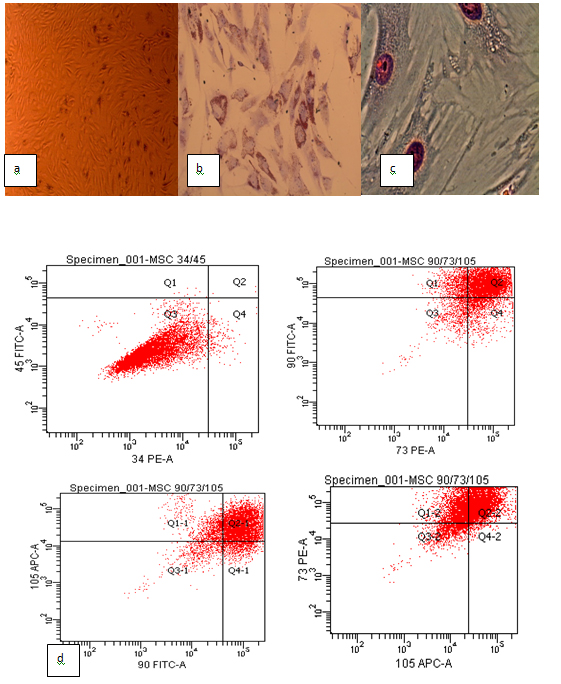
Figure 1.Primary culture, phenotypic characterization and differentiating capacity of mice mesenchymal stem cells. (a) Mesenchymal stem cells in the primary culture; (b) Oil red O staining showed lipid vacuoles for adipogenic differentiation; (c) Alizarin red staining showed calcium deposition for osteogenic differentiation; (d) The phenotypic profile of mesenchymal stem cells. The mesenchymal stem cells were positive for CD73, CD90 and CD105, negative for CD34 and CD45.
Flow cytometry confirmed the characteristic expressions of mesenchymal stem cells related to surface markers. The mouse MSCs were 94.5% positive for CD73, 95% positive for CD90, 94.6% positive for CD105, 98.5% negative for CD34, and 97.8% negative for CD45 Figure 1d).
The capacity of the MSCs to differentiate into osteocytes and adipocytes was demonstrated in vitro. The cells that were maintained in adipocytes differentiation condition for 2 weeks exhibited lipid vacuoles in the colonies as indicated by oil red O stain Figure 1b). MSCs that were conditioned in osteogenic differentiation media for 2 weeks exhibited calcium deposition as indicated by alizarin red S stain Figure 1c).
Growth Factors and Cytokines in MSC-Conditioned Media
Levels of growth factors and cytokines were measured in MSC-CM using ELISA. Different levels of the IL-1β, TNF-α, IL-2, IL-4, IL-6, IL-10, INF-γ, GM-CSF, VEGF and NO were found in MSC-CM Table 1).
Outcome |
Levels |
IL-1β level (pg/mL) |
237.8 ± 6.2 |
IL-2 level (pg/mL) |
1.6 ± 0.1 |
IL-4 level (pg/mL) |
0.4 ± 0.1 |
IL-6 level (pg/mL) |
2.3 ± 0.4 |
IL-10 level (pg/mL) |
1.5 ± 0.2 |
TNF-α level (pg/mL) |
527.8 ± 24.9 |
IFN-γ level (pg/mL) |
99.2 ± 0.8 |
VEGF level (pg/mL) |
128.6 ± 12.7 |
GM-CSF level (pg/mL) |
0.7 ± 0.2 |
NO level (μM/mL) |
12.01 ± 1.6 |
Table 1. Levels of Cytokine and Growth Factors in Mesenchymal Stem Cells-Conditioned Media.
IL: Interleukin; TNF-α: Tumor Necrosis Factor-alpha; IFN-γ: Interferon-gamma; VEGF: Vascular Endothelial Growth Factor; GM-CSF: Granulocyte/Macrophage-Colony Stimulating Factor; NO: Nitric Oxide.
Induction of diabetes mellitus in the animal model
The animal remained hyperglycemic post-STZ infusion over the study time period higher than 15 mM/L Table 2). There was no mortality post-STZ treatment. The blood glucose levels were checked by glucometer. These results suggested that intraperitoneal administration of STZ induce Diabetes Mellitus in B6 mice
Day |
Glucose (mM/L) |
0 |
4.98 ± 0.35 |
6 |
10.33 ± 2.46 (p<0.05) |
9 |
15.08 ± 1.83 (p<0.05) |
12 |
19.37 ± 1.02 (p<0.05) |
28 |
19.03 ± 0.92 (p<0.05) |
Table 2. Blood glucose levels in STZ-induced Diabetes Mellitus male mice
Data are presented as mean ± standard error of mean; P, compared with glucose level on day 0.
MSCs Grafts and MSC-CM Injection Promote Healing of Wounds
The burned wounds were prepared on the dorsum of intact B6 male mice and in STZ-induced Diabetes Mellitus B6 male mice. Control animals received identical burned wounds without MSCs or MSC-CM engraftment to measure baseline healing. Engraftment with MSCs and injection MSC-CM helped discriminate between the effect of local delivery of cells and the subsequent systemic response. The response on the grafted animals revealed direct, cell-mediated effect of engraftment, whereas the response on the injection MSC-CM revealed the extent of the indirect systemic effect of MSC-CM injection on promoting wound healing Table 3).
Day |
PBS wounds |
MSCs wounds |
MSC-CM wounds |
Wound healing in intact B6 male mice |
0 |
1.46 ± 0.1 |
1.46 ± 0.1 |
1.46 ± 0.1 |
7 |
2.15 ± 0.4 |
1.3 ± 0.2* |
1.67 ± 0.1* |
14 |
1.2 ± 0.2 |
0,81 ± 0.1* |
1.0 ± 0.2* |
21 |
0.68 ± 0.3 |
0.5 ± 0.2* |
0.58 ± 0.3* |
24 |
0.36 ± 0.5 |
0,005 ± 0.005* |
0.06 ± 0.05* |
30 |
0.1 ± 0.1 |
0.0 ± 0.0 |
0.0 ± 0.0 |
Wound healing in STZ-induced Diabetes Mellitus B6 male mice |
0 |
1.46 ± 0.1 |
1.46 ± 0.1 |
1.46 ± 0.1 |
7 |
1.86 ± 0.3 |
0.71 ± 0.2* |
1.1 ± 0.3* |
14 |
1.36 ± 0.5 |
0.47 ± 0.4* |
0.74 ± 0.5* |
21 |
0.95 ± 0.4 |
0.15 ± 0.1* |
0.4 ± 0.2* |
24 |
0.5 ± 0.3 |
0.01 ±0.01* |
0.05 ± 0.03* |
30 |
0.15 ± 0.05 |
0.0 ± 0.0 |
0.0 ± 0.0 |
Table 3. Wound closer at different time points
PBS wounds: Phosphate Buffer Saline treated group; MSCs wounds: Mesenchymal Stromal Cells treated group; MSC-CM wounds: Mesenchymal Stromal Cell-Conditioned Media treated group; *p<0.05, compared with PBS-treated group.
Accelerated healing persisted in the MSCs and MSC-CM wounds through days 21 and 24 in intact mice, and through days 7 and 21 in STZ-induced Diabetes Mellitus mice. We found that the MSCs or MSC-CM wounds exhibited significantly improved closure in normal healing and in diabetic mice p<0.05) at time point, days, 7, 14, 21, and 24 Table 2).
Next, we examined the impact of MSCs engraftment or MSC-CM injection on reepithelialization. As shown in Figure 2, the epithelial thicker showed no differences among these groups on day 30. However, the epithelial was thicker in all experimental groups compare normal skin.
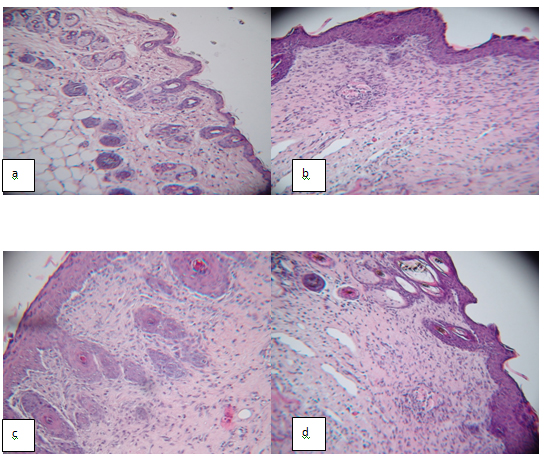
Figure 2. Engraftment of BM-MSCs and MSC-Conditioned Media into wound bed in STZ-induced Diabetes Mellitus B6 male mice promote wound healing. 106 MSCs in 200 μL PBS (n=8) or 200 μL CM (n=8) were inoculated into the wound area in diabetic mice 0 days after wound induction. Control group were treated with injection of PBS (n=8). Histological HE staining of sections showed that the wound healing in STZ-induced Diabetes Mellitus mice was incomplete in saline treated group. (a) Normal skin; (b) PBS treated wounds; (c) MSCs treated wounds; (d) MSC-CM treated wounds.
These findings suggested that MSCs or MSC-CM may participate in and promote the formation of derma by topical wound bed transplantation.
We also evaluated the impact of MSCs or MSC-CM on hair follicle, sebaceous gland, and blood vessels formation in wound Table 4).
Outcome |
Control |
PBS wounds |
MSCs wounds |
MSC-CM wounds |
hair follicle |
6.80 ± 1.10 |
0.40 ± 0.55*,@,% |
3.05 ± 2.38 |
3.88 ± 1.64* |
sebaceous gland |
8.20 ± 1.30 |
1.20 ± 0.45*,@,% |
2.25 ± 0.50* |
3.75 ± 1.91* |
blood vessels |
11.20 ± 4.44 |
0.60 ± 0.55*,@,% |
2.50 ± 1.00* |
anks Irina Kim for help with phenotype characterization of BM-MSCs.
References
- Zhang J, Guan M, Xie C, Luo X, Zhang Q, et al. (2014) Increased growth factors play a role in wound healing promoted by noninvasive oxygen-ozone therapy in diabetic patients with foot ulcers. Oxid Med Cell Longev 2014: 273475. [Crossref]
- Sena CM, Bento CF, Pereira P, Seiça R (2010) Diabetes mellitus: new challenges and innovative therapies. EPMA J 1: 138-163. [Crossref]
- Tuttolomondo A, Maida C, Pinto A (2015) Diabetic foot syndrome: Immune-inflammatory features as possible cardiovascular markers in diabetes. World J Orthop6:62-67. [Crossref]
- Tecilazich F, Dinh T, Pradhan-Nabzdyk L, Leal E, Tellechea A, et al. (2013) Role of endothelial progenitor cells and inflammatory cytokines in healing of diabetic foot ulcers. PLoS One8: e83314. [Crossref]
- Tang Y, Zhang MJ, Hellmann J, Kosuri M, Bhatnagar A, et al. (2013) Proresolution therapy for the treatment of delayed healing of diabetic wounds. Diabetes62: 618-627. [Crossref]
- Nuschke A (2014) Activity of mesenchymal stem cells in therapies for chronic skin wound healing. Organogenesis10: 29-37. [Crossref]
- Demidova-Rice TN, Hamblin MR, Herman IM (2012) Acute and Impaired Wound Healing: Pathophysiology and Current Methods for Drug Delivery, Part 2: Role of Growth Factors in Normal and Pathological Wound Healing: Therapeutic Potential and Methods of Delivery. Adv Skin Wound Care25:349-370. [Crossref]
- Cao Z, Zhang G, Wang F, Liu H, Liu L, et al. (2013) Protective effects of mesenchymal stem cells with CXCR4 up-regulation in a rat renal transplantation model. PLoS One8: e82949. [Crossref]
- Gutierrez-Fernandez M, Rodriguez-Frutos B, Ramos-Cejudo J, Otero-Ortega L, Fuentes B, et al. (2013) Effect of intravenous administration of allogenic bone marrow- and adipose tissue-derived mesenchymal stem cells on functional recovery and brain repair markers in experimental ischemic stroke. Stem Cell Res Ther4:11-26. [Crossref]
- Si Y, Zhao Y, Hao H, Liu J, Guo Y, et al. (2012) Infusion of mesenchymal stem cells ameliorates hyperglycemia in type 2 diabetic rats: identification of a novel role in improving insulin sensitivity. Diabetes61: 1616-1625. [Crossref]
- Jiang Y, Zhang Y, Zhang L, Wang M, Zhang X, et al. (2014) Therapeutic effect of bone marrow mesenchymal stem cells on laser-induced retinal injury in mice. Int J Mol Sci15: 9372-9385. [Crossref]
- Pileggi A (2012) Mesenchymal stem cells for the treatment of diabetes. Diabetes61: 1355-1356. [Crossref]
- Yeung TY, Seeberger KL, Kin T, Adesida A, Jomha N, et al. (2012) Human mesenchymal stem cells protect human islets from pro-inflammatory cytokines. PLoS One7: e38189. [Crossref]
- Ricciardi M, Malpeli G, Bifari F, Bassi G, Pacelli L, et al. (2012) Comparison of epithelial differentiation and immune regulatory properties of mesenchymal stromal cells derived from human lung and bone marrow. PLoS One7: e35639. [Crossref]
- Wan J, Xia L, Liang W, Liu Y, Cai Q (2013) Transplantation of bone marrow-derived mesenchymal stem cells promotes delayed wound healing in diabetic rats. J Diabetes Res2013: 647107. [Crossref]
- Allen RJ Jr, Soares MA, Haberman ID, Szpalski C, Schachar J, et al. (2014) Combination therapy accelerates diabetic wound closure. PLoS One9: e92667. [Crossref]
- Bernardi S, Severini GM, Zauli G, Secchiero P (2012) Cell-based therapies for diabetic complications. Exp Diabetes Res2012: 872504. [Crossref]
- O’Loughlin A, Kulkarni M, Creane M, Vaughan E, Mooney E, et al. (2013) Topical Administration of Allogeneic Mesenchymal Stromal Cells Seeded in a Collagen Scaffold Augments Wound Healing and Increases Angiogenesis in the Diabetic Rabbit Ulcer. Diabetes62:2588-2594. [Crossref]
- Wan J, Cai Q, Liu Y (2013) Effect of different transplantations with bone-marrow derived mesenchymal stem cells on diabetic foot ulcers in rats. Zhong Nan Da Xue Xue Bao Yi Xue Ban38: 347-355. [Crossref]
- Yeum CE, Park EY, Lee SB, Chun HJ, Chae GT (2013) Quantification of MSCs involved in wound healing: use of SIS to transfer MSCs to wound site and quantification of MSCs involved in skin wound healing. J Tissue Eng Regen Med7: 279-291. [Crossref]
- Pastar I, Stojadinovic O, Yin NC, Ramirez H, Nusbaum AG, et al. (2014) Epithelialization in Wound Healing: A Comprehensive Review. Adv Wound Care (New Rochelle)3: 445-464. [Crossref]
- Mishra PJ, Mishra PJ, Banerjee D (2012) Cell-free derivatives from mesenchymal stem cells are effective in wound therapy. World J Stem Cells4: 35-43. [Crossref]
- Shin L, Peterson DA (2013) Human Mesenchymal Stem Cell Grafts Enhance Normal and Impaired Wound Healing by Recruiting Existing Endogenous Tissue Stem/Progenitor Cells. Stem Cells Transl Med 2:33-42. [Crossref]
- Chen L, Tredget EE, Wu PY, Wu Y (2008) Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One3: e1886. [Crossref]
- Hoch AI, Binder BY, Genetos DC, Leach JK (2012) Differentiation-dependent secretion of proangiogenic factors by mesenchymal stem cells. PLoS One7: e35579. [Crossref]
- Melief SM, Geutskens SB, Fibbe WE, Roelofs H (2013) Multipotent stromal cells skew monocytes towards an anti-inflammatory interleukin-10-producing phenotype by production of interleukin-6. Haematologica98: 888-895. [Crossref]
- Pradhan Nabzdyk L, Kuchibhotla S, Guthrie P, Chun M, Auster ME, et al. (2013) Expression of neuropeptides and cytokines in a rabbit model of diabetic neuroischemic wound healing. J Vasc Surg58: 766-775. [Crossref]
- Boomsma RA, Geenen DL (2012) Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis. PLoS One7: e35685. [Crossref]
- Beyazyıldız E, Pınarlı FA, Beyazyıldız O, Hekimoğlu ER, Acar U, et al. (2014) Efficacy of topical mesenchymal stem cell therapy in the treatment of experimental dry eye syndrome model. Stem Cells Int 2014: 250230. [Crossref]
- Baraniak PR, McDevitt TC (2010) Stem cell paracrine actions and tissue regeneration. Regen Med5: 121-143. [Crossref]
- Rennert RC, Sorkin M, Januszyk M, Duschar D, Kosaraju R, et al. (2014) Diabetes impairs the angiogenic potential of adipose-derived stem cells by selectively depleting cellular subpopulations. Stem Cell Res Ther5:79-94. [Crossref]
Editorial Information
Editor-in-Chief
Masayoshi Yamaguchi
Emory University School of Medicine
Article Type
Research Article
Publication history
Received: December20, 2015
Accepted: January 14, 2016
Published: January 16, 2016
Copyright
©2016Lykov AP.This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Lykov AP, Bondarenko NA, Poveshchenko OV, Miller TV, Poveshchenko AF, et al.(2016) Biomedical cellular product for wound healing. Integr ObesityDiabetes. 2:doi: 10.15761/IOD.1000139
Corresponding author
Alexander P. Lykov
Scientific Institute of Clinical and Experimental Lymphology, Novosibirsk, Russian Federation
E-mail : aplykov2@mail.ru
Outcome |
Levels |
IL-1β level (pg/mL) |
237.8 ± 6.2 |
IL-2 level (pg/mL) |
1.6 ± 0.1 |
IL-4 level (pg/mL) |
0.4 ± 0.1 |
IL-6 level (pg/mL) |
2.3 ± 0.4 |
IL-10 level (pg/mL) |
1.5 ± 0.2 |
TNF-α level (pg/mL) |
527.8 ± 24.9 |
IFN-γ level (pg/mL) |
99.2 ± 0.8 |
VEGF level (pg/mL) |
128.6 ± 12.7 |
GM-CSF level (pg/mL) |
0.7 ± 0.2 |
NO level (μM/mL) |
12.01 ± 1.6 |
Table 1. Levels of Cytokine and Growth Factors in Mesenchymal Stem Cells-Conditioned Media.
IL: Interleukin; TNF-α: Tumor Necrosis Factor-alpha; IFN-γ: Interferon-gamma; VEGF: Vascular Endothelial Growth Factor; GM-CSF: Granulocyte/Macrophage-Colony Stimulating Factor; NO: Nitric Oxide.
Day |
Glucose (mM/L) |
0 |
4.98 ± 0.35 |
6 |
10.33 ± 2.46 (p<0.05) |
9 |
15.08 ± 1.83 (p<0.05) |
12 |
19.37 ± 1.02 (p<0.05) |
28 |
19.03 ± 0.92 (p<0.05) |
Table 2. Blood glucose levels in STZ-induced Diabetes Mellitus male mice
Data are presented as mean ± standard error of mean; P, compared with glucose level on day 0.
Day |
PBS wounds |
MSCs wounds |
MSC-CM wounds |
Wound healing in intact B6 male mice |
0 |
1.46 ± 0.1 |
1.46 ± 0.1 |
1.46 ± 0.1 |
7 |
2.15 ± 0.4 |
1.3 ± 0.2* |
1.67 ± 0.1* |
14 |
1.2 ± 0.2 |
0,81 ± 0.1* |
1.0 ± 0.2* |
21 |
0.68 ± 0.3 |
0.5 ± 0.2* |
0.58 ± 0.3* |
24 |
0.36 ± 0.5 |
0,005 ± 0.005* |
0.06 ± 0.05* |
30 |
0.1 ± 0.1 |
0.0 ± 0.0 |
0.0 ± 0.0 |
Wound healing in STZ-induced Diabetes Mellitus B6 male mice |
0 |
1.46 ± 0.1 |
1.46 ± 0.1 |
1.46 ± 0.1 |
7 |
1.86 ± 0.3 |
0.71 ± 0.2* |
1.1 ± 0.3* |
14 |
1.36 ± 0.5 |
0.47 ± 0.4* |
0.74 ± 0.5* |
21 |
0.95 ± 0.4 |
0.15 ± 0.1* |
0.4 ± 0.2* |
24 |
0.5 ± 0.3 |
0.01 ±0.01* |
0.05 ± 0.03* |
30 |
0.15 ± 0.05 |
0.0 ± 0.0 |
0.0 ± 0.0 |
Table 3. Wound closer at different time points
PBS wounds: Phosphate Buffer Saline treated group; MSCs wounds: Mesenchymal Stromal Cells treated group; MSC-CM wounds: Mesenchymal Stromal Cell-Conditioned Media treated group; *p<0.05, compared with PBS-treated group.
Outcome |
Control |
PBS wounds |
MSCs wounds |
MSC-CM wounds |
hair follicle |
6.80 ± 1.10 |
0.40 ± 0.55*,@,% |
3.05 ± 2.38 |
3.88 ± 1.64* |
sebaceous gland |
8.20 ± 1.30 |
1.20 ± 0.45*,@,% |
2.25 ± 0.50* |
3.75 ± 1.91* |
blood vessels |
11.20 ± 4.44 |
0.60 ± 0.55*,@,% |
2.50 ± 1.00* |
2.75 ± 0.89* |
Table 4. The hair follicle, sebaceous gland, and blood vessels formation in wound in STZ-induced Diabetes Mellitus mice in burned wound
PBS wounds: Phosphate Buffer Saline treated group; MSCs wounds: Mesenchymal Stromal Cells treated group; MSC-CM wounds: Mesenchymal Stromal Cell-conditioned media treated group; *compared with control p<0.05; @compared with MSCs treated group p<0.05; %compared with MSC-CM treated group p<0.05.

Figure 1.Primary culture, phenotypic characterization and differentiating capacity of mice mesenchymal stem cells. (a) Mesenchymal stem cells in the primary culture; (b) Oil red O staining showed lipid vacuoles for adipogenic differentiation; (c) Alizarin red staining showed calcium deposition for osteogenic differentiation; (d) The phenotypic profile of mesenchymal stem cells. The mesenchymal stem cells were positive for CD73, CD90 and CD105, negative for CD34 and CD45.

Figure 2. Engraftment of BM-MSCs and MSC-Conditioned Media into wound bed in STZ-induced Diabetes Mellitus B6 male mice promote wound healing. 106 MSCs in 200 μL PBS (n=8) or 200 μL CM (n=8) were inoculated into the wound area in diabetic mice 0 days after wound induction. Control group were treated with injection of PBS (n=8). Histological HE staining of sections showed that the wound healing in STZ-induced Diabetes Mellitus mice was incomplete in saline treated group. (a) Normal skin; (b) PBS treated wounds; (c) MSCs treated wounds; (d) MSC-CM treated wounds.
Articles
 
|


