It has been reported that Cav2.1-mediated N-methyl-d-aspartate (NMDA) receptor signaling is critical pathway in hippocampus and nucleus accumbens for spatial short-term memory. Neuronal voltage-dependent Cav2.1 and Cav2.2 have predominantly expressions at presynaptic neuronal terminals and mediate glutamate release in the central nervous systems. Recently, although Cav2.2 in the hippocampus and nucleus accumbens is also critical for spatial cognition, it remains unknown that functional Cav2.2-mediated NMDA receptor signaling in cognitive performance at the system level. This study examined whether Cav2.2-mediated NMDA receptor signaling mediates spatial short-term memory using the Y-maze test via a combined subthreshold doses of pharmacological approach. In previous our studies, Mice received systemic injection of NMDA receptor blocker (+/–)-3-(2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP, 5mg/kg), mice received intra-hippocampal injection of Cav2.2 blocker (ω-conotoxin GVIA, 1pg/side), or mice received intra-accumbens injection of Cav2.2 blocker (ω-conotoxin GVIA, 1pg/side) were ineffective for the spatial short-term memory. However, a combination of subthreshold doses of 5mg/kg CPP systemic injection and 1pg/side ω-conotoxin GVIA intra-hippocampal injection triggered a spatial short-term memory deficit. Furthermore, a combination of subthreshold doses of 5mg/kg CPP systemic injection and 1pg/side ω-conotoxin GVIA intra-accumbens injection also showed impaired spontaneous alternation patterns. These results indicate that Cav2.2-mediated NMDA receptor signaling is critical in the hippocampus and nucleus accumbens for spatial short-term memory. These subthreshold pharmacological approach presented here is easily performed and can be used to study functional signaling pathways in various regions.
Cav channel, ω-Conotoxin GVIA, CPP, Subthreshold dose, Y maze test
Neuronal voltage-dependent Ca2+ channel (VDCC), including Cav2.1 (P/Q-type), Cav2.2 (N-type), and Cav2.3 (R-type) channels, mediate the presynaptic machinery involved in the vesicular release of neurotransmitters [1-3]. A VDCC is a molecular complex comprising α1, α2-δ, β, and γ subunits [3]. The α1 subnuit is essential for channel functioning and determines fundamental channel properties [3]. The ~190kDa pore-forming transmembrane α1 subunit (~2000 amino acids) is organized in four homologous domains (I–IV), comprising six transmembrane α helices (S1–S6) and the pore-forming P loop between S5 and S6.
It has been reported that N-methyl-D-aspartate (NMDA)-dependent processes are involved in the mechanisms of memory [4] and that glutamatergic system is one of the neurotransmitter systems regulated by Cav2.1 and Cav2.2 [5,6]. It has been also reported that glutamatergic afferents from the hippocampus provide the main source of information to the nucleus accumbens (NAc) during cognitive activity [7-11]. Our previous reports have showed that Cav2.1-regulated glutamatergic signaling in the hippocampus [12] and NAc [13] is important in short-term spatial learning. We have also demonstrated the importance of Cav2.2-regurated signaling in the hippocampus [14] and NAc [15] for spatial short-term memory. However, the role of Cav2.2-regurated glutamatergic signaling in the neural circuits underlying spatial short-term memory has not been studied.
In previous study, subthreshold doses of cannabinoid CB1 receptor antagonist and selective serotonin reuptake inhibitors, which separately had no effect on antidepressant behavioral tests, showed a clear effect in combination without the side effects [16], suggesting that combinations of compounds with different molecular targets are useful tools to study the neuronal signaling mechanisms.
In this study, to examine the importance of Cav2.2-mediated glutamatergic transmission in the hippocampus and NAc for spatial short-term memory, we administered the Y-maze test for mice treated with ω-conotoxin GVIA, Cav2.1 inhibitor and (+/–)-3-(2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP), a NMDA receptor antagonist.
Animals
All animal procedures were approved by the Animal Experiments Committee of Shanghai Jiao Tong University and RIKEN. The C57BL/6J mice were provided by Charles River Japan (Kanagawa, Japan). The mice were given free access to water and food pellets (CRF-1; Oriental Yeast Co. Ltd., Tokyo, Japan) and were housed under a 12/12-h light/dark cycle (lights on from 08:00 to 20:00) at 23 ± 1°C and 55 ± 5% humidity. Testing was performed during the light phase of the cycle. We used separate groups of male 2-month-old mice for each of the behavioral tests. All experiments were conducted blind to the treatment condition of the mouse.
Drugs
CPP (5mg/kg, Sigma-Aldrich) was dissolved in 0.9% NaCl (vehicle) and injected intraperitoneally 20min before behavioral testing. Cav2.2 blocker, ω-conotoxin GVIA (1, 10, or 50 pg/μL, Peptide Institute, Osaka, Japan) were dissolved in 0.9% NaCl (vehicle). Mice that were not treated with drugs received an equivalent volume of vehicle.
Infusion
Under anesthesia and using standard stereotaxic procedures, stainless-steel guide cannulae (22-gauge) were implanted into the dorsal hippocampus (posterior to bregma, -2.0mm; lateral to midline, ±2.0mm; ventral from the dura, −2.0mm) or nucleus accumbens (+1.7mm, ±1.0 mm, +2.3 mm). Mice were allowed to recover for at least 1 week following surgery. The mice were briefly anesthetized with isoflurane to facilitate insertion of the injection cannula (26-gauge). Infusions into the hippocampus (0.1μL/side) or nucleus accumbens (0.1μL/side) are accomplished at a rate of 0.05μL/min 30min before behavioral testing. The injection cannula was left in place for 2min following the infusion.
Y maze tests
All behavioral tests were conducted between 10:00 and 16:00 by a trained experimenter who was blind to the mouse strains. We used separate groups of male mice for each of the behavioral tests. The mice were moved into the behavioral testing room at least 2h before testing. The Y-maze test was performed using the reported procedure with slight modifications [12]. The experiments were performed at 35 lux. Before behavioral testing, the mice were placed in one of the compartments and allowed to move freely on the one of the arms for 10min. Each mouse performed one trial. An arm entry was defined as three legs entering one of the arms, and the sequence of entries was recorded manually using videotapes. An alteration was defined as entry into all three arms with consecutive choices. The percentage of spontaneous alteration was calculated as (actual alteration/maximum alteration) × 100.
Histology
Histological verification of the cannula locations was performed at the end of behavioral testing. Mice were perfused transcardially with 0.9% saline, followed by 4% PFA. After extraction from the skull, the brains were postfixed in 4% PFA and then transferred to a 30% sucrose solution until sectioning. Coronal sections (40μm thick, taken every 120μm) were cut on a cryostat (–16°C) and mounted on glass microscope slides. After drying, the sections were stained with cresyl violet. Mice with injection needle placements outside of the boundaries of targeted areas were excluded from behavioral analysis.
Data analysis
Data are presented as means ± standard error on the mean (SEM). Statistical analyses for the behavioral tests were conducted using Excel Statistics 2006 (SSRI, Tokyo, Japan). Data were analyzed using repeated measures ANOVA with Tukey’s test.
The drug doses were determined according to previous report [14,15,17]. In our previous study, we examined four groups of mice, including four groups each of mice that were given systemic injections of 0, 2.5, 5, or 10mg/kg CPP. Although the groups did not differ significantly in the total number of arm entries, spontaneous alterations were significantly different [17]. The mice given 10mg/kg CPP showed fewer spontaneous alteration behaviors than the mice given 5mg/kg CPP. We used 5mg/kg as a subthreshold dose of CPP in this study. On the other hand, among mice received intra-hippocampal or intra-accumbens injection of 0, 1, 5, or 10pg/side ω-conotoxin GVIA, mice received 5 or 10pg/side ω-conotoxin GVIA in both regions triggered a spatial short-term memory deficit [14,15]. To examine the effect of subthreshold doses of ω-conotoxin GVIA on Y-maze test, we injected 0, 0.1, 1, or 5pg/side ω-conotoxin GVIA into hippocampus or NAc.
Effects of subthreshold doses of CPP plus intra-hippocampal injection of ω-conotoxin GVIA on Y-maze test
We examined four groups of mice (n=10 each), including group each of mice that were given intra-hippocampal injections of 0, 0.1, 1, or 5pg/side ω-conotoxin GVIA plus 5mg/kg CPP, respectively. The groups did not differ significantly in arm entries [F(3,36)=0.8, P>0.05] (data not shown) (Figure 1A), while spontaneous alterations were significantly different [F(3,36)=122.2, P<0.01] (Figure 1B). The mice given 1pg/side ω-conotoxin GVIA plus 5mg/kg CPP showed fewer spontaneous alteration behaviors than the mice given 0pg/side ω-conotoxin GVIA plus 5mg/kg CPP.
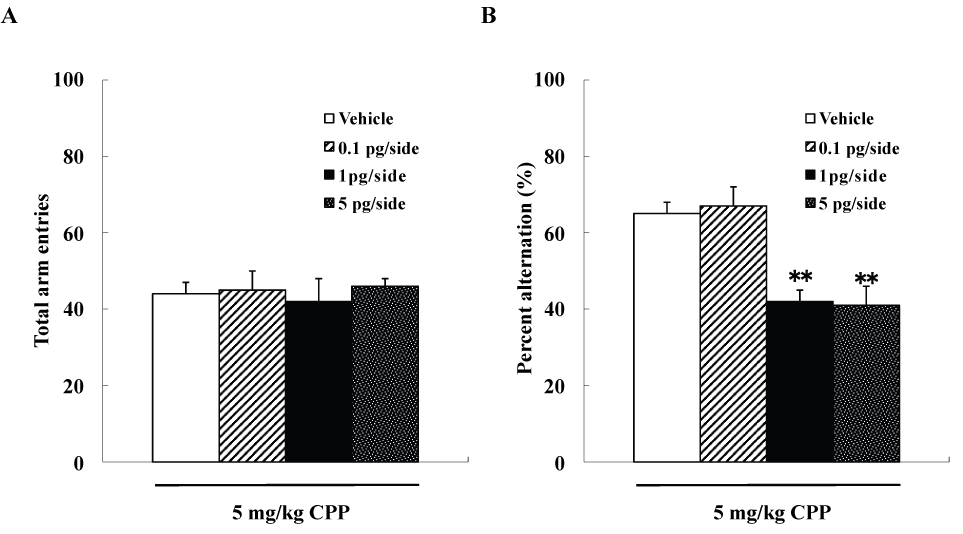
Figure 1. Effects of subthreshold doses of systemic injection CPP plus intra-hippocampal injection of ω-conotoxin GVIA on Y-maze test. **P<0.01 compared to the control groups (Tukey’s test).
Effects of subthreshold doses of CPP plus intra-accumbens injection of ω-conotoxin GVIA on Y-maze test
We examined four groups of mice (n=10 each), including group each of mice that were given intra-accumbens injections of 0, 0.1, 1, or 5pg/side ω-conotoxin GVIA plus 5mg/kg CPP, respectively. The groups did not differ significantly in arm entries [F(3,36)=0.7, P>0.05] (Figure 2A), while spontaneous alterations were significantly different [F(3,36)=112.2, P<0.01] (Figure 2B). The mice given 1pg/side ω-conotoxin GVIA plus 5mg/kg CPP showed fewer spontaneous alteration behaviors than the mice given 0pg/side ω-conotoxin GVIA plus 5mg/kg CPP.
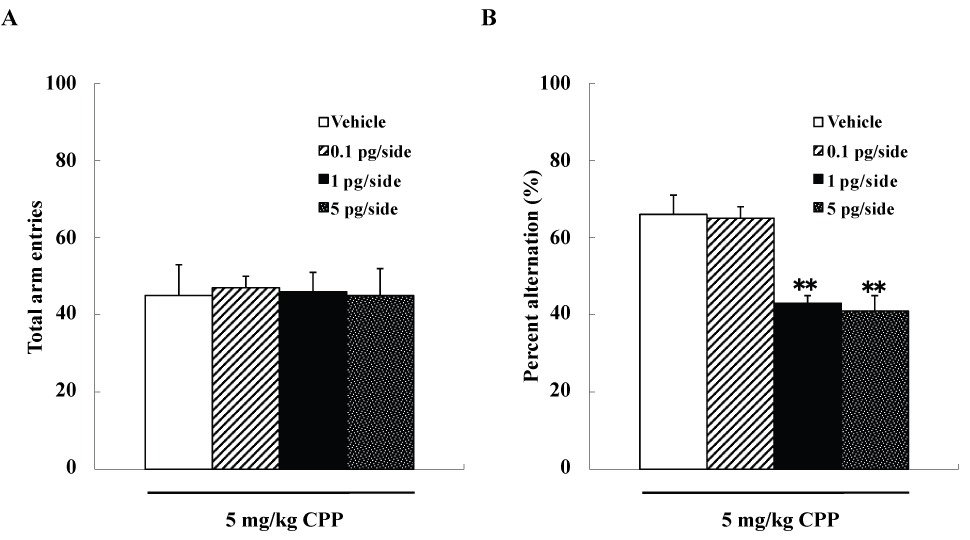
Figure 2 . Effects of subthreshold doses of systemic injection CPP plus intra-accumbens injection of ω-conotoxin GVIA on Y-maze test. **P<0.01 compared to the control groups (Tukey’s test).
In this study, to evaluate whether CaV2.2-mediated NMDA receptor signaling has an important role in memory, we administered the Y-maze tests for spatial memory to mice treated with a combination of subthreshold doses of systemic injection of NMDA receptor CPP and local injection of Cav2.2 blocker, ω-conotoxin GVIA. The Y-maze test is based on the spontaneous tendency of rodents to enter a novel arm more often than the other arms due to the spatial short-term memory [18]. Behavioral stimuli such as electrical stimulation in a fear-conditioning test are used to examine memory formation in rodents [4]. Since CPP and ω-conotoxin GVIA are thought to be effect on analgesia [19,20], cognitive tests without stimulations would be more appropriate for this study. The administration of different drugs at a lower dose induced the effects of phenotypes and identified functional signaling pathways [16], because the precise neuronal circuit systems play an important role in phenotypes at behavioral levels.
Pharmacological studies have showed that NMDA receptor antagonists impair the cognitive functions [21-23] and that NMDA receptor agonists enhance memory tasks [24]. Previous studies have demonstrated that systemic injection of CPP dose-dependently inhibited spatial short-term memory in the Y maze test [17]. Since Cav2.2 and NMDA receptor are present at a variety of synapses [25,26], we need to examine which sites are important for spatial short-term memory formation. In this regard, studies using local injection into specific region are a useful tool to specifically understand the relevant neuronal circuits. Indeed, local injections have showed that Cav2.1-regulated glutamatergic signaling in the hippocampus [12] and NAc [13] is important in short-term spatial learning.
We showed that a subthreshold dose of CPP significantly decreased the spontaneous alteration behaviors when combined with a subthreshold dose of ω-conotoxin GVIA, although administration of CPP or ω-conotoxin GVIA alone was ineffective in the Y maze test [14,15,17]. The precise regulation of Ca2+ signaling through Cav2.2 plays an important role in neuronal circuits [3]. Therefore, because Cav2.2 on the presynapse is important in glutamate release [3] and NMDA receptor on the postsynapse is important in signaling leading to cognitive function [4], the combination of subthreshold doses induced impairment of glutamatergic circuit system and deficit of spatial short-term memory. Furthermore, these results were detected in a combination of subthreshold doses of CPP systemic injection plus ω-conotoxin GVIA intra-hippocampal injection and a combination of subthreshold doses of CPP systemic injection plus ω-conotoxin GVIA intra-accumbens injection. It has been reported that glutamatergic transmission within the NAc plays a central role in the transfer of different types of information from cortical and limbic regions including hippocampus [7-11]. Our results indicate that spatial short-term memory formation is impaired when either Cav2.2-mediated NMDA receptor signaling in hippocampus or NAc is inhibited. On the other hand, previous reports have showed that Cav2.1-regulated glutamatergic signaling in the hippocampus [12] and NAc [13] is important in short-term spatial learning. We need a future study to understand the role of different VDCCs in the neural circuits underlying spatial short-term memory
In conclusion, we found that subthreshold doses ofω-conotoxin GVIA and CPP in the hippocampus and NAc, which are effective when administered separately at threshold doses, have a combined effect on spatial short-term memory. These results indicate that Cav2.2-mediated NMDA receptor signaling is critical in the hippocampus and nucleus accumbens for spatial short-term memory. The subthreshold pharmacological approach would be useful to analyze specifically functional signaling cascade in neuronal circuit. Our results also indicate that the detailed comparison of combinations of different pharmacological agents, concentrations and infusion sites would be helpful in understanding the relationships among the synaptic signaling, neuronal circuit and observed behavior.
2021 Copyright OAT. All rights reserv
The authors declare no competing interests.
WL and ET designed and supervised the research, and wrote the manuscript. YZ and KN performed the surgeries and behavioral experiments. All authors read and approved the final version of the manuscript.
- 1. Evans RM, Zamponi.GW (2006) Presynaptic Ca2+ channels-integration centers for neuronal signaling pathways. Trends Neurosci 29: 617-624. [Crossref]
- 2. Jarvis S, Zamponi GW (2007) Trafficking and regulation of neuronal voltage-gated calcium channels. Curr Opin Cell Biol 19: 474-482. [Crossref]
- 3. Catterall WA, Few AP (2008) Ca2+ channel regulation and presynaptic plasticity. Neuron 59:882-901. [Crossref]
- 4. Zhou Y, Takahashi E, Li W, Halt A, Wiltgen B, et al. (2007) Interactions between the NR2B receptor and CaMKII modulate synaptic plasticity and spatial learning. J Neurosci 27: 13843-13853. [Crossref]
- 5. Kimura M, Katayama K, Nishizawa Y (1999) Role of glutamate receptors and voltage-dependent calcium channels in glutamate toxicity in energy-compromised cortical neurons. Jpn J Pharmacol 80: 351-358. [Crossref]
6. Lee I, Hunsaker MR, Kesner RP (2005) The role of hippocampal subregions in detecting spatial novelty. Behav Neurosci 119: 145-153. [Crossref]
- 7. Di Ciano P, Cardinal RN, Cowell RA, Little SJ, Everitt BJ (2001) Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of pavlovian approach behavior. J Neurosci 21: 9471-9477. [Crossref]
- 8. Floresco SB, Seamans JK, Phillips AG (1996) Differential effects of lidocaine infusions into the ventral CA1/subiculum or the nucleus accumbens on the acquisition and retention of spatial information. Behav Brain Res 81: 163-171. [Crossref]
- 9. Friedman DP, Aggleton JP, Saunders RC (2002) Comparison of hippocampal, amygdala, and perirhinal projections to the nucleus accumbens: combined anterograde and retrograde tracing study in the Macaque brain. J Comp Neurol 450: 345-365. [Crossref]
- 10. Sargolini F, Roullet P, Oliverio A, Mele A (1999) Effects of lesions to the glutamatergic afferents to the nucleus accumbens in the modulation of reactivity to spatial and non-spatial novelty in mice. Neuroscience 93: 855-867. [Crossref]
- 11. Sargolini F, Roullet P, Oliverio A, Mele A (2003) Effects of intra-accumbens focal administrations of glutamate antagonists on object recognition memory in mice. Behav Brain Res 138: 153-63. [Crossref]
- 12. Takahashi E, Niimi K, Itakura C (2010) Impairment of spatial short-term memory following acute administration of the NMDA receptor antagonist in heterozygous rolling Nagoya mice carrying the Cav2.1α1 mutation. Behav Brain Res 213: 121-125.
- 13. Takahashi E, Niimi K, Itakura C (2011) Role of Cav2.1-mediated NMDA receptor signaling at nucleus accumbens in spatial short-term memory. Behav Brain Res 218: 353-356.
- 14. Zhou Y, Niimi K, Li W, Takahashi E (2015) Effects of Cav2.2 inhibitor on hippocampal spatial short-term cognition. Integr Mol Med 2: 95-98.
- 15. Zhou Y, Niimi K, Li W, Takahashi E (2015) Disruption of spatial cognition by intra-accumbens injection of Cav2.2 inhibitor. Integr Mol Med 2: 109-111.
- 16. Takahashi E, Katayama M, Niimi K, Itakura C (2008) Additive subthreshold dose effects of cannabinoid CB(1) receptor antagonist and selective serotonin reuptake inhibitor in antidepressant behavioral tests. Eur J Pharmacol 589: 149-156. [Crossref]
- 17. Takahashi E, Niimi K, Itakura C (2010) Subthreshold pharmacological and genetic approaches to analyzing Cav2.1-mediated NMDA receptor signaling in short-term memory. Eur J Pharmacol 645: 113-118. [Crossref]
- 18. Granon S, Save E, Buhot MC, Poucet B (1996) Effortful information processing in a spontaneous spatial situation by rats with medial prefrontal lesions. Behav Brain Res 78: 147-154. [Crossref]
- 19. Ferber J, Juniewicz H, Głogowska E, Wroński J, Abraszko R, et al. (2003) Tramadol for postoperative analgesia in intracranial surgery. Its effect on ICP and CPP. Neurol Neurochir Pol 34: 70-79. [Crossref]
- 20. Fukuizumi T, Ohkubo T, Kitamura K (2003) Spinally delivered N-, P/Q- and L-type Ca2+-channel blockers potentiate morphine analgesia in mice. Life Sci 73: 2873-2881. [Crossref]
- 21. Bischoff C, Tiedtke PI (1993) Competitive and non-competitive NMDA receptor antagonists in spatial learning tasks. Eur J Pharmacol 213: 269-273. [Crossref]
- 22. Maurice T, Su TP, Parish DW, Nabeshima T, Privat A (1994) PRE-084, a selective PCP derivative, attenuates MK-801-induced impairment of learning in mice. Pharmacol Biochem Behav 49: 859-869. [Crossref]
- 23. Parada-Turska J, Turski WA (1990) Excitatory amino acid antagonists and memory: effect of drugs acting at N-methyl-D-aspartate receptors in learning and memory tasks. Neuropharmacology 29: 1111-1116. [Crossref]
- 24. Sarter M, Bodewitz G, Stephens DN (1988) Attenuation of scopolamine-induced impairment of spontaneous alternation behavior by antagonist but not inverse agonist and agonist-carbolines. Psychopharmacology 94: 491-495. [Crossref]
- 25. Tanaka O, Sakagami H, Kondo H (1995) Localization of mRNAs of voltage-dependent Calcium channels: four subtypes of alpha 1- and beta-subunits in developing and mature rat brain. Brain Res Mol Brain Res 30: 1-16. [Crossref]
- 26. Paoletti P (2011) Molecular basis of NMDA receptor functional diversity. Eur J Neurosci 33: 1351-1365. [Crossref]


