Abstract
Background
Gaps in the literature exist related to our understanding of the relationship between leptin and adiponectin concentrations with energy expenditure in prepubertal children. The Biochemical Evaluation during a Health Intervention Programme (B.E. H.I.P.) study aimed to investigate the impact of high intensity exercise on adipocytokines in overweight and obese male and female children.
Objective
We assessed the impact of a 10-week high intensity exercise programme on adipocytokine concentrations in prepubertal obese children.
Research design and methods
A pre-post test crossover design (N=27; control=11; intervention=16) was employed and 25 overweight children between 5 and 10 years of age completed the 10-week intervention of progressive exercise. Fasting blood samples were analysed for leptin, tumour necrosis factor-alpha (TNF-α), Interleukin-6 (IL-6), insulin, adiponectin, soluble leptin receptor (SLR) and glucose concentration. All variables were repeatedly sampled with up to four time points between the pre- and post-intervention analysis and between the post-intervention and post-study analysis.
Results
Leptin had a mean concentration decrease of 7.77 ng/mL in the intervention participants and this change was significantly more pronounced in males than females (p=0.05). Although there was no significant change in the SLR (p=0.9762), males did have a significantly greater concentration increase than the negative change in females (p=0.04). The FLI also significantly decreased from 2.00 to 1.55 for all intervention participants; males had a greater decrease than females (p=0.01). Adiponectin significantly increased by 6.07 ng/mL in all intervention participants, but there was no significant sex difference (p=0.28). There were no further significant sex differences in adipocytokines, insulin, glucose or HOMA score.
Conclusions
A progressive high intensity exercise program demonstrated increased adiponectin and decreased leptin concentrations in overweight and obese children.
Key words
leptin, adiponectin, pediatric, obesity, exercise, high intensity, body composition
Introduction
Obesity is associated with numerous acute and chronic health problems and early mortality. The 2004 Canadian Community Health Survey (CCHS) reported that 2-17 year olds have overweight and obesity rates of 26 and 8%, respectively and these overweight rates have more than doubled, while the obesity rates have tripled, in the past 25 years [1]. Specifically, overweight Canadians aged 7-13 were estimated to be 33 and 26% for males and females, respectively, while the obesity prevalence rate was approximately 20% [2]. Numerous comorbities are associated with obesity; therefore it is important to develop novel modalities to reduce these increasing rates.
A means of identifying risk for obesity is through the use of a biomarker, such as leptin or adiponectin. These are the two most abundant adipocyte-derived hormones, or adipocytokines, in healthy humans [3,4]. They are important in the regulation of body composition and the low-grade inflammatory state associated with obesity. Additional studies are required to identify the potential utility of these biomarkers to elucidate their role in identifying and monitoring children suited for a specific intervention [5]. The outcomes of these studies will better clarify the potential role for obesity biomarkers [6].
Important physiological evidence of a regulatory system controlling body weight occurred with the discovery of the gene associated with obesity, the ob gene, and its protein leptin [7]. Since its discovery, leptin has been reported to function within the long-term energy balance system and to regulate fat and glucose metabolism [8]. Leptin has a positive correlation with body mass index (BMI) and fat mass, a negative correlation with fat-free mass and is usually higher in obese relative to lean child and adolescent populations. This is seen in both healthy [9-11] and obese [12-14] children and adolescents. Furthermore, studies on leptin and its receptor have provided evidence for their regulation of appetite, thermogenesis and metabolism [8]. Given the role of leptin in modulating long-term energy intake and its positive correlation with fat mass, it may have clinical utility as a biomarker to identify children at risk of becoming overweight or obese.
The leptin receptor (Ob-R) interacts directly with the leptin protein. The Ob-R exists in several isoforms due to alternative splicing and each isoform has a large extracellular, transmembrane and variable intracellular domain [15]. Ob-Re is the soluble leptin receptor (SLR) and the major leptin binding protein in blood [16]. The examination of the SLR concentration is important because the separate concentrations of total, free and bound leptin could each play a key role on their own [6]. Individuals with obesity have a significantly elevated leptin to SLR ratio [17,18]. When SLR concentrations decrease, there would be higher concentrations of liberated free leptin and relatively higher than normal circulating leptin concentration with obesity. A decrease in SLR concentrations could indicate a decrease in functional Ob-R and be a sign of leptin resistance [18-20]. It remains unclear from the literature how free leptin concentration changes in prepubertal children. Furthermore, the influence of physical activity intensity in prepubertal children on both total and free leptin concentration changes remains to be determined. It has been recommended to develop exercise intervention programmes with a variety of longer bouts of exercise at greater intensities, in addition to accounting and controlling for caloric intake [6].
Four independent research groups discovered adiponectin in the mid-1990s [21-24]. It is the most specific and abundant transcript found in adipocytes [22] and is generally lower in obese versus nonobese children, adolescents and adults [25-28]. Experimental evidence suggests that adiponectin has an important role in increasing insulin sensitivity, regulating energy homeostasis and has anti-inflammatory and anti-atherogenic properties [29-33]. It is an important molecular link between obesity, insulin resistance and atherogenic lipoproteins [34].
Weight loss is known to increase adiponectin concentration. This was seen in obese 6-15 year olds that underwent a one year programme of low intensity physical exercise, nutrition education and behaviour therapy [35]. The children and adolescents that showed a significant weight loss had a significant increase in adiponectin concentrations. By contrast, those with no significant weight loss had no significant change in their adiponectin concentration. The adiponectin increase was also shown in a group of obese adolescents following a weight reduction programme [36]. The moderate restriction of energy intake and regular moderate-to-high intensity physical activity in obese adolescents induced beneficial changes in body weight and composition, lipid oxidation and blood parameters [36]. It remains unclear if these interventions will have the same results when examining only overweight and obese prepubertal children, as the direct impact of energy expenditure on adiponectin in these children is not well known.
There currently exists a gap in the literature related to our understanding of the relationship between leptin and adiponectin concentrations with energy expenditure in prepubertal children. The Biochemical Evaluation during a Health Intervention Programme (B.E. H.I.P.) study aimed to investigate the impact of high intensity exercise on adipocytokines in overweight and obese male and female children. Specifically, this paper addressed the impact of a 10-week high intensity exercise programme on adipocytokine concentrations in prepubertal obese children.
Materials and methods
Study design and participants
The study methodology, programme description and detailed intervention have been previously described [37]. The intervention portion of B.E. H.I.P. (Biochemical Evaluation during a Health Intervention Programme) included two physical activity sessions each week, three nutrition sessions and two parent sessions. The following paragraphs will outline the design, exercise programme and intervention.
A pre-post test crossover design was employed and included a 10-week intervention. Group A took part in the intervention initially, while Group B was monitored as the control group. After the first 10 weeks of the intervention, Group A became the follow-up group and Group B took part in the intervention. The participants recruited for B.E. H.I.P were prepubertal overweight or obese male and female children between the ages of 5 and 10 years from families throughout Calgary, Alberta, Canada. A total of 27 children enrolled in this study. There were 16 participants in Group A and 11 participants in Group B (Table 1). Two participants in Group B dropped out of the study before the end of the intervention due to personal family issues beyond the scope of the study. Parental consent and child ascent were obtained for all participants and appropriate standards for human experimentation were followed. The University Research Ethics Board at the University of Calgary approved the research protocol.
|
|
Group A+a(Mean ± SD)
|
Group Bb (Mean ± SD)
|
p-value
|
|
Age (years) All
|
8.5 ± 1.5
|
8.8± 1.1
|
0.5615
|
|
Female
|
8.1 ± 1.6
|
9.0 ± 1.7
|
0.4158
|
|
Male
|
9.4 ± 0.9
|
8.8 ± 0.9
|
0.2262
|
|
Weight (kg) All
|
51.6 ± 15.3
|
50.1± 10.1
|
0.7750
|
|
Female
|
48.4 ± 15.7
|
50.6 ± 6.3
|
0.8265
|
|
Male
|
58.5 ± 13.2
|
49. 9 ± 11.5
|
0.2412
|
|
Height (cm) All
|
138.0± 10.7
|
142.7± 9.6
|
0.2461
|
|
Female
|
134.8 ± 10.9
|
148.7 ± 8.5
|
0.0650
|
|
Male
|
145.0 ± 6.7
|
140.5 ± 9.5
|
0.3797
|
|
WC (cm) All
|
80.0± 10.6
|
76.6± 7.5
|
0.4076
|
|
Female
|
78.6 ± 11.4
|
72.9 ± 4.5
|
0.4196
|
|
Male
|
82.0 ± 9.3
|
77.9 ± 8.2
|
0.9773
|
Table 1. Participant Characteristics at Time of Entry into the Study.
+: Crossover;an = 16 (F = 11; M = 5);bn = 11 (F = 3; M = 8); WC = waist circumference
The exercise programme centred on a progressive increase in high intensity exercise at each session for a total of twenty minutes by week ten. High intensity was defined as a heart rate that was ≥ 75% of the participant’s HRmax [38]. Physical activity was quantitatively assessed during each exercise session using an Actiheart monitor (Respironics, Inc/Mini Mitter Co., Bend, Oregon) or Polar heart rate monitor (RS400, Polar Electro Canada Inc, Lachine, Quebec). Two instructors certified by the Alberta Fitness Leadership Certification Association facilitated the exercise classes.
Physical health and body composition assessment
All participants obtained height (HT), weight (WT) and waist circumference (WC). These measures were completed in light clothing and were carried out by a Certified Exercise Physiologist. The child’s percent fat mass (%FM), percent fat free mass (%FFM), trunk fat and bone mineral density (BMD) were obtained from the DEXA instrument (Hologic QDR 4500A scanner, Hologic Inc, Waltham, MA) (Table 2).
Table 2. All Variables Collected for the Child and Parent.
|
|
Participant Traits
|
Adiposity Measurements
|
Blood Measurements
|
Other*
|
|
Child
|
HT, WT, age, HR, BP
|
WC, BMI Percentile, DEXA
|
TG, TC, HDL-C, LDL-C, TC:HDL-C, leptin, SLR, IL-6, TNF-α, adiponectin, insulin, glucose
|
Muscular strength and flexibility, LSI, 3-Day Diet Record
|
|
Parent
|
HT, WT, age, FEAH
|
WC, BMI
|
TC
|
|
HT = height, WT = weight, HR = heart rate; BP = blood pressure; WC = waist circumference, DEXA = dual energy x-ray absorptiometry; BMI = body mass index; TG = triglyceride; TC = total cholesterol; HDL-C = high-density lipoprotein; LDL-C = low-density lipoprotein; SLR = soluble leptin receptor; IL-6 = interleukin 6; TNF-α = tumour necrosis factor-alpha; LSI = leisure survey index; FEAH = Family Eating and Activity Habits Questionnaire
Blood sample analysis
The homeostasis model of assessment (HOMA) determined the participant’s insulin sensitivity [(insulin (µU/mol) X glucose (mmol/L)) / 22.5] [39]. The free leptin index (FLI) was defined as leptin /SLR.
Serum leptin, tumour necrosis factor-alpha (TNF-α), Interleukin-6 (IL-6), and insulin were analyzed with a human serum ELISA adipokine Lincoplex kit (Linco Research, St. Charles, Missouri). Manufacturer reported intra- and inter-assay variation was 1.4-7.9% and < 21%, respectively. The reported sensitivity for leptin, TNF-α, insulin and IL-6 were 85.4 pg/mL, 0.14 pg/mL, 50.9 pg/mL and 1.6 pg/mL, respectively. Adiponectin concentration was analyzed with a human ELISA kit (Alpco Diagnostics, Salem, New Hampshire). The manufacturer determined limit of detection was 100 pg/mL, the intra-assay precision was between 3.84% (1.86 μg/mL) and 2.97% (23.36 μg/mL) and the inter-assay precision (coefficient of variation) was between 5.15 (2.5 μg/mL) and 2.84% (24.82 μg/mL). Similarly, the soluble leptin receptor utilized a human ELISA (Bio Vendor, Candler, North Carolina). The manufacture determined limit of detection was 0.4 ng/mL, intra-assay precision ranged from 6.5-10.0% and the inter-assay precision ranged between 4.2-9.9%. The human ELISA for insulin concentration (Linco Research, St. Charles, Missouri) had a manufacture determined limit of detection of 2 μU/mL, intra-assay precision of 6.94% (7.0 μU/mL) and 4.64% (120.5 μU/mL) and inter-assay precision of 10.2% (6.2 μU/mL) and 9.1% (120.9 μU/mL). Glucose concentrations were determined with a glucose trinder assay (Stanbio Laboratory, Boerne, TX). The manufacturer reported coefficient of variation for within-day precision was 1.6% (15.56 ± 1.11 mmol/L) and 1.2% (17.33 ± 3.4 mmol/L), while between-day precision was 3.0% (15.56 ± 1.11 mmol/L) and 2.0% (17.33 ± 3.4 mmol/L).
Statistical analyses
All continuous variables were examined for normality. Leptin, FLI and HOMA had skewed distributions. Natural log transformations led to more normal distributions for these variables therefore all of their analyses are completed on the natural log scale. BMI-percentile and the energy expenditure and activity counts from the Actiheart monitors were also not normally distributed (highly skewed left); these variables did not become normalized with transformations and required nonparametric analyses.
Results were reported as means ± standard deviation (SD), except for the variables that required nonparametric analysis. These were described by medians ± 25th and 27th interquartile range (IQR). Comparisons between variables at baseline, pre-intervention versus post-intervention and post-intervention versus post-study were analyzed by Student’s t tests for continuous variables using paired and unpaired conditions, as appropriate. Similarly, nonparametric analysis utilized the Wilcoxon signed-rank test for matched pair analysis, while the Mann-Whitney U test (Wilcoxon rank-sum) compared two independent groups. Analysis of covariance, with sex as a cofactor, was also completed for continuous variables.
All variables including blood markers, body composition and basic participant characteristics were repeatedly sampled. There were up to four time points between the pre- and post-intervention analysis and between the post-intervention and post-study analysis. These were analyzed by response feature analysis to account for the repeated measurement of the variable within one individual. Response feature analysis, or two-stage analysis, reduces the multiple responses on each participant to a single biologically meaningful response that concisely characterizes data structure [40]. In the current study, the mean slope from the four testing points was determined for each participant with each continuous variable of interest. Response feature analysis is a straightforward approach for analyzing repeated measures data, manages well missing data and is possible to interpret the significance of the slope change by Student’s t test [40]. The t test in this study assessed if the mean slope change was significantly different: 1) from zero within the intervention or control group, by each group for all participants and by each group while controlling for sex and 2) from zero for all intervention participants, for each sex separately and while controlling for sex. The Student’s t test was replaced by the Wilcoxon signed-rank test or the Mann-Whitney U test for matched pair analysis or two independent sample analysis, respectively, when nonparametric analyses were required.
Pearson correlation coefficients (r) and Spearman rank correlation coefficients (rho) were determined to look at linear association between leptin or adiponectin and the additional blood, participant and body composition variables. Subsequently, a multivariate stepwise linear regression model for the dependent variable of leptin and adiponectin were completed for all individuals completing the intervention (n=25). The independent variables had to have a partial correlation that was at least at a significant level of 0.15 to enter into the model.
The statistical significance level of a p-value (p) ≤ 0.05 was utilized. Trends were described when the p-value ≤ 0.15. All statistics were completed with Intercooled Stata 9.2 (StataCorp, College Station, Texas).
Results
Body composition
Results for baseline versus post-intervention values for all participants that completed the intervention were described in Table 3. There were no significant mean changes from the pre- to post-intervention time points between sexes except for weight (p=0.0103).
Table 3. Body Composition Results for All Intervention Participants
|
|
n
|
Pre-intervention (Mean ± SD)
|
Post-Intervention (Mean ± SD)
|
Mean Change
|
P-Value
|
|
WT
|
25
|
52.3 ± 13.3
|
52.6 ± 13.1
|
0.34
|
0.1918
|
|
HT
|
25
|
140.7 ±10.9
|
142.6 ± 10.9
|
1.88
|
<0.0001
|
|
WC
|
25
|
79.6 ± 9.5
|
78.5 ± 9.6
|
-1.06
|
0.0631
|
|
BMI %lea
|
25
|
98.8 (96.4, 99.2)
|
98.5 (96.2, 99.1)
|
N/A
|
<0.0001
|
|
BMD
|
25
|
0.80 ± 0.1
|
0.81 ± 0.1
|
0.01
|
0.0998
|
|
%FM
|
25
|
40.2 ± 4.6
|
38.1 ± 4.8
|
-2.17
|
<0.0001
|
|
%FFM
|
25
|
57.6 ± 4.4
|
59.7 ± 4.6
|
2.18
|
<0.0001
|
a = median (interquartile range); WT = weight (kg); HT = height (cm); WC = waist circumference (cm); BMI %le = body mass index-for-age percentile; BMD = bone mineral density (g/cm2); %FM = fat mass (%); %FFM = fat-free mass (%)
The response feature analysis established significant differences for percent fat and fat-free mass when comparing the intervention group (n=16) versus the control group (n=11) over the four time points throughout the intervention. The intervention group had a %FM mean slope change of -0.17, while the control group had -0.02 change (p=0.0005). %FFM had a positive mean slope change of 0.20 in the intervention group, versus the 0.02 slope change in the control group (p=0.006).
Adipocytokines, Glucose and HOMA
Table 4 described the direct pre- and post-intervention changes. Changes for leptin and adiponectin were shown in Figure 1 and 2. Leptin had a mean concentration decrease of 7.77 ng/mL in the intervention participants. This change was significantly more pronounced in males than females (p=0.05). Although there was no significant change in the SLR (p=0.9762), males did have a significantly greater concentration increase than the negative change in females (p=0.04). The FLI also significantly decreased from 2.00 to 1.55 for all intervention participants; males had a greater decrease than females (p=0.01). Adiponectin significantly increased by 6.07 ng/mL in all intervention participants, but there was no significant sex difference (p=0.28). There were no further significant sex differences in adipocytokines, insulin, glucose or HOMA score.
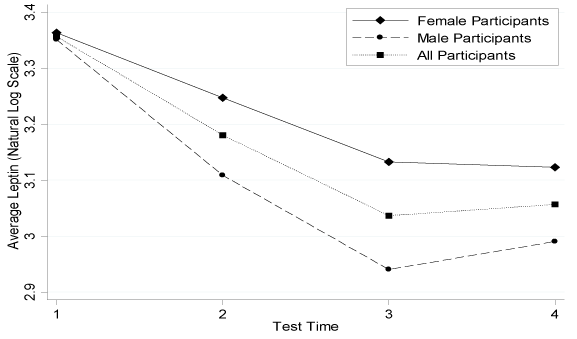
Figure 1.Average Leptin Concentration Change during the Intervention .n=25 for all participants; n=13 for females; n=12 for males
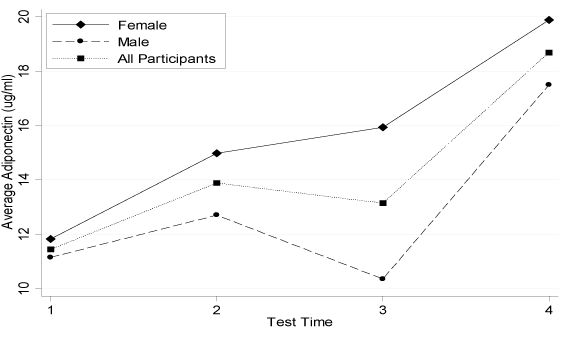
Figure 2.Average Adiponectin Concentration Change during the Intervention.n=25 for all participants; n=13 for females; n=12 for males
Table 4. Adipocytokine Results for All Intervention Participants
|
|
n
|
Pre-intervention (Mean ± SD)
|
Post-intervention (Mean ± SD)
|
Mean Change
|
P-Value
|
|
Leptin (ng/mL) All
|
25
|
32.62± 21.8
|
24.84± 15.1
|
-7.77
|
<0.0001
|
|
Female
|
13
|
31.89 ± 25.8
|
27.19 ± 19.2
|
-4.70
|
0.0233
|
|
Male
|
12
|
33.40 ± 17.6
|
22.30 ± 9.1
|
-11.1
|
0.0003
|
|
SLR (ng/mL) All
|
25
|
20.64± 7.8
|
20.62± 7.8
|
-0.02
|
0.9762
|
|
Female
|
13
|
19.49± 7.5
|
17.92± 7.7
|
-1.57
|
0.1865
|
|
Male
|
12
|
21.89± 8.2
|
23.54± 7.0
|
1.65
|
0.0885
|
|
Adiponectin (ug/mL) All
|
25
|
13.07± 4.5
|
19.15± 5.5
|
6.07
|
<0.0001
|
|
Female
|
13
|
15.01± 5.0
|
20.15± 6.0
|
5.14
|
0.0006
|
|
Male
|
12
|
10.97± 3.0
|
18.06± 5.0
|
7.09
|
0.0003
|
|
TNF-α (pg/mL) All
|
25
|
6.05± 2.7
|
5.4± 2.1
|
-0.63
|
0.0453
|
|
Female
|
13
|
6.63± 3.2
|
5.54± 1.9
|
-1.09
|
0.0311
|
|
Male
|
12
|
5.42± 1.9
|
5.29± 2.3
|
-0.12
|
0.7262
|
|
FLI All
|
25
|
2.00 ± 2.1
|
1.55± 1.5
|
-0.46
|
0.0088
|
|
Female
|
13
|
1.94± 2.1
|
1.95± 1.8
|
0.01
|
0.7951
|
|
Male
|
12
|
2.04± 2.1
|
1.12± 9.8
|
-0.92
|
0.0014
|
|
HOMA All
|
23
|
2.54± 1.0
|
1.98± 1.0
|
-0.57
|
0.0008
|
|
Female
|
12
|
2.68± 1.1
|
2.27± 1.2
|
-0.41
|
0.0517
|
|
Male
|
11
|
2.40 ± 0.9
|
1.66 ± 0.6
|
-0.74
|
0.0078
|
|
Glucose (mmol/L) All
|
24
|
4.47± 0.6
|
4.04± 0.5
|
-0.43
|
0.0096
|
|
Female
|
12
|
4.37± 0.6
|
4.03± 0.4
|
-0.34
|
0.1773
|
|
Male
|
11
|
4.58± 0.7
|
4.05± 0.6
|
-0.54
|
0.0210
|
SLR = soluble leptin receptor; TNF-α = tumour necrosis factor-alpha; FLI = free
leptin index; HOMA = homeostasis model of assessment
The response feature analysis illustrated significant mean slope changes in the primary outcome variables of this study, leptin and adiponectin, when comparing the intervention versus the control group (Table 5). In addition to leptin being significantly different between the groups (p=0.03), there was also a statistically significant mean slope difference between the leptin concentration change for males in the intervention versus control group (-0.03 ± 0.02 versus 0.001 ± 0.03, respectively; p=0.04). The positive adiponectin mean slope change in the intervention group was significantly different than the negative mean slope change of the control group (Figure 3). In addition, females in the intervention group had a significantly more positive slope than females in the control group (0.42 ± 0.34 versus -0.38 ± 0.38; p=0.012). Males in the intervention group showed a trend for a more positive mean slope change from zero than did the males in the control group (0.24 ± 0.5 versus -0.16 ± 0.23; p=0.095).
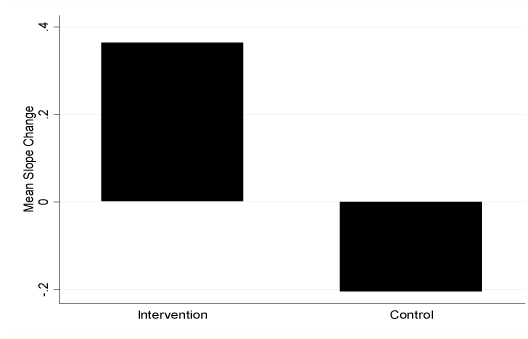
Figure 3.Mean Slope Change of Adiponectin Concentration fortheIntervention and Control Groups. a*=p of 0.0021 (positive mean slope change for the intervention group was significantly different from zero); b*=p of 0.0008 (mean slope changes for the intervention and control groups were signifcantly different from each other); c*=p of 0.0446 (negative mean slope change for the group was significantly different from zero)
Table 5. Mean Slope Change of Adipocytokine Results during the Intervention: Within and Between Group Comparison.
|
|
Group
|
Slope
(Mean ± SD)
|
Slope
(95% CI)
|
Difference from Zero (p-value)
|
Group Difference (p-value)
|
|
Leptin
|
Intervention
|
-0.02 ± 0.03
|
-0.03, -0.002
|
0.0264
|
0.0301
|
|
Control
|
0.01 ± 0.03
|
-0.01, 0.03
|
0.3272
|
|
SLR
|
Intervention
|
-0.12 ± 0.35
|
-0.31, 0.07
|
0.1956
|
0.8619
|
|
Control
|
-0.21 ± 0.53
|
-0.61, 0.20
|
0.2761
|
|
Adiponectin
|
Intervention
|
0.36 ± 0.39
|
0.15, 0.57
|
0.0021
|
0.0008
|
|
Control
|
-0.21 ± 0.23
|
-0.41, -0.06
|
0.0446
|
|
TNF-α
|
Intervention
|
-0.08 ± 0.19
|
-0.18, 0.02
|
0.0924
|
0.1378
|
|
Control
|
0.03 ± 0.15
|
-0.08, 0.14
|
0.5897
|
|
FLI
|
Intervention
|
-0.01 ± 0.04
|
-0.03, 0.01
|
0.2639
|
0.1002
|
|
Control
|
0.02 ± 0.04
|
-0.01, 0.04
|
0.2329
|
SLR = soluble leptin receptor; TNF-α = tumour necrosis factor-alpha; FLI = free leptin Index
Glucose was only assayed pre-and post-intervention, so response feature analysis could not be completed. The intervention group (n=15) had a significant decrease in glucose concentration from pre- to post-intervention (4.59 ± 0.6 mmol/L to 3.95 ± 0.6 mmol/L; p=0.004). The control group (n=9) did not change (4.25 ± 0.7 mmol/L to 4.21 ± 0.4 mmol/L; p=0.87). The difference between groups was significant (0.67 ± 0.29 mmol/L; p=0.03). To the analysis according to sex showed that females in the intervention group decreased by 0.56 mmol/L (n=10; p=0.03), while females in the control group increased by 0.76 mmol/L (n=2; p=0.03).
Post-intervention response feature analysis was completed on Group A (n=13) for the 11-week follow-up. The negative mean slope change in adiponectin was significantly different from zero (-0.42 ± 0.58; p=0.024). Female adiponectin change differed significantly from zero (n=8; -0.65 ± 0.59; p=0.017), while males did not (n=5; -0.04 ± 0.3; p=0.82). These sex differences were significantly different (p=0.06). No other significant changes occurred post-intervention.
2021 Copyright OAT. All rights reserv
Correlation and multivariate regression analyses
Significant correlations were found between: 1) adiponectin change and BMD change (correlation coefficient=0.43, p=0.03), 2) leptin change with sex (correlation coefficient=0.39, p=0.048) and 3) leptin change with SLR change (correlation coefficient=-0.39, p=0.05). The interaction between heart rate change and leptin occurred with the partial correlation of sex as a covariate (correlation coefficient=-0.36, p=0.09).
The multivariate regression model with adiponectin as the dependent variable found that only BMD remained a significant independent predictor (Figure 4; β coefficient=110.95, R2=0.186, p=0.032). Leptin multivariate analysis with and without fat mass (kg) was completed because the correlation coefficient showed a trend toward significance (p=0.162). Free FLI and SLR were not included, due to their high inter-dependence with the leptin concentration. The model without fat mass included sex as the only significant independent predictor for leptin (β coefficient=0.213, R2=0.162, p=0.046). The second model with fat mass revealed that only fat mass was an independent predictor for leptin (β coefficient=0.136, R2=0.201, p=0.024).
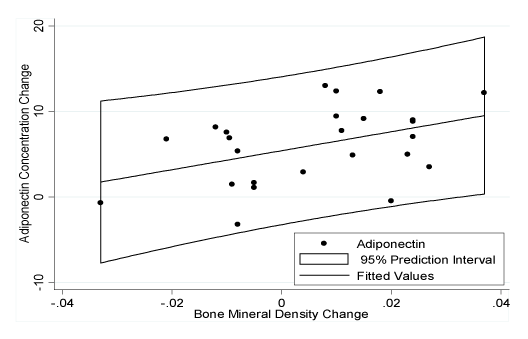
Figure 4. Relationship between the Change in Adiponectin Concentration and Bone Mineral Density Concentration during the Intervention
Discussion
To date our understanding of the impact of high intensity exercise interventions in prepubertal obese children has been limited. This successful 10-week intervention programme elicited favourable changes in the concentrations of leptin and adiponectin. Furthermore, there was a decrease in percent fat mass and trunk fat mass and an increase in percent fat-free mass. Additional beneficial secondary changes included decreases in TNF-α, FLI, HOMA and glucose concentrations. Leptin concentration and soluble leptin receptor decreased in males to a greater extent than in females.
The efficacy of exercise as a treatment strategy in overweight children and adolescents is not as well-established as it is in adults [41]. In a commentary by Gutin [42], he supported that vigorous activity, as opposed to energy intake restriction, was the optimal mechanism for reducing childhood obesity. This was strengthened by a study that showed children performing five minutes or less of vigorous activity were 4.0 or 2.9 times more likely to have ≥ 20% or 25% body fat, respectively, compared to children performing fifteen or more minutes of less of vigorous activity each day [43]. This further supported the importance of utilizing high intensity exercise in intervention and prevention programmes; the B.E. H.I.P. study was able to systematically evaluate this hypothesis and the results supported this contention [37]. The decrease in leptin concentration and highly significant increase in adiponectin support that the B.E. H.I.P. programme was associated with marked improvements in the physiological profile of the children. These hormone changes, in addition to body composition, TNF-α, FLI, HOMA and glucose changes, coincide with the significant increase in high intensity exercise completed by these children (23.88 to 35.56 minutes; [37]. There is a paucity of studies utilizing high intensity exercise as an intervention tool specifically in prepubescent children and the B.E. H.I.P. study was able to help fill this knowledge gap.
The concentrations of leptin in the overweight and obese children of the B.E. H.I.P. study were higher in comparison to other published concentrations for children that are not overweight or obese [44,45]. Leptin positively correlated to percent fat mass and negatively correlated to percent fat-free mass at the intervention baseline. A major factor influencing plasma leptin concentrations in humans is adipose tissue mass [46], therefore a higher concentration was expected in the B.E. H.I.P. overweight and obese children. The change in leptin concentration during the B.E. H.I.P. intervention was independently predicted by both body fat and sex, however body fat and sex were not interdependent. Although a positive relationship between leptin concentration and percent fat mass does exist in overweight and obese children, it is hypothesized that the participation in high intensity exercise can have independent effects on the concentration of leptin. The effects favourably decrease leptin concentrations to a greater extent than is explained by fat mass change alone. This was supported by the significantly greater duration in high intensity exercise for males in the B.E. H.I.P. intervention [37]. In addition, the change in average heart rate was significantly influenced by leptin and sex, as determined by multivariate analysis [37]. The actual mechanism to describe how high intensity exercise and greater energy expenditure decreased leptin concentration remains unknown. It is hypothesized that the interaction between exercise intensity and leptin may be due to the influence of bone and muscle development through the greater mechanical stimulation in high intensity exercise and the interactions between leptin and adiponectin [19]. BMD and leptin were correlated, although BMD did not independently predict leptin concentration. It has been proposed that the importance of sufficient nutrient intake for growth may require a positive energy balance, in addition to adequate mechanical tissue stimulation for correct nutrient partitioning into fat and fat-free tissues [42]. As reviewed by Rosen and Bouxsein [47], and examined in mice by Rubin et al.[48], greater stimulation directs stem cell differentiation into bone and muscle, as opposed to fat. Subsequently, the mechanical stimulation would be induced by high intensity exercise and not energy intake restriction [42].
The majority of articles examining leptin do not differentiate between total, free and bound leptin concentration in the blood. Although this study could not directly examine free leptin concentrations, it was able to determine the concentrations of leptin and the SLR, which together provided the FLI. Higher SLR concentrations are found in lean compared to obese adults [18]. Similarly, obese children and adolescents have higher leptin, but lower SLR concentrations relative to lean controls [19,20]. The SLR concentrations found during B.E. H.I.P. were lower, while the leptin concentrations were higher than the previously published work in children [20]. These differences may be attributed to differences in the types of assays used between the studies, as has been found in leptin concentration analysis, whereby RIA generates lower concentrations than ELISA [45]. It has been postulated that when leptin binds to the SLR, leptin degradation and clearance may be delayed from circulation [49]. This would increase the concentration of available circulating leptin to bind to tissue receptors. Furthermore, SLR concentrations have been found to increase and stabilize in obese children when they have a significant reduction in weight, relative to children with little weight loss [20]. The current study found a trend for increasing SLR concentrations in males, but there was a non-significant decrease in females. There was also a significant decrease in the FLI in all participants, but particularly in males. The favourable changes in males may have been due to their higher exercising intensity. The B.E. H.I.P. intervention did modestly alter the FLI to a lower, more favourable value.
Pre- and post-intervention adiponectin concentrations significantly increased by 6.07 μg/ml. This change is highly significant, both statistically and clinically. The concentrations determined in B.E. H.I.P. participants fall within a previously reported range of 4.2 – 20.8 μg/ml in a child population with a wide total range of BMI-for-age percentiles from 16.9 to 99.4 [50]. The increasing concentrations of adiponectin throughout the intervention were clinically significant and they opposed the unhealthy decline in concentration observed in the control group. The use of adiponectin as a potential biomarker for evaluating lifestyle intervention programmes was supported by this study and a 2008 study in prepubertal and pubertal children with mixed BMI [51]. Although a direct cause and effect relationship cannot be ascertained between greater energy expenditure and adiponectin in the B.E. H.I.P. study, these changes remain biologically significant and support the success of the intervention. Few known studies in children have found such a significant mean change for prepubertal children that are overweight and obese, particularly without significant weight lose. Increases have been previously reported in obese children that had significant weight loss [35]. A further study with a larger group of children that are both normal and overweight should be initiated. These children should be randomly assigned to different exercise intensity groups to determine if similar effects occurred with varying levels of energy expenditure.
Adiponectin accumulates in injured blood vessel walls and dose-dependently inhibits TNF-α signaling within human aortic endothelial cells and decreases the production of TNF-α in macrophage, as summarized by Matsubara et al. [52]. The inhibition of TNF-α would decrease the ability of TNF-α to stimulate endothelial cell activation, promote adhesion molecule synthesis and monocyte adhesion to the arterial endothelium, which is critical to the development of vascular disease [52]. Furthermore, adiponectin has attenuated phagocytic activity in cultured macrophages and reduced TNF-α production [53]. The significant TNF-α decrease in B.E. H.I.P. intervention participants was likely due, in part, to its interactions with adiponectin changes. More specifically, the control group actually had a positive slope change in TNF-α (non-significant), while adiponectin had a significant negative slope. These changes were opposite to that of the intervention group. The small, negative correlation between the change in adiponectin and TNF-α during this study would support the more favourable anti-inflammatory environment that the high intensity exercise programme provided.
There are a lack of lifestyle intervention programmes that have examined both leptin and adiponectin in overweight and obese children [54]. A recent study utilizing a lifestyle intervention programme with moderate physical activity reported favourable changes in body fat, HOMA, blood lipids and leptin, but no changes in adiponectin concentration in obese adolescent females [54]. It aimed to have exercising heart rates between 55 and 75% of the child’s predicted maximum heart rate, which was monitored by a Polar monitor. The actual exercising heart rates were not reported. They found body fat change scores (mean=-3.6%) that were similar to B.E. H.I.P. (mean=2.2%). Park et al. [54] only found a significant leptin concentration decrease of 3.0 ng/ml, while B.E. H.I.P found a decrease of 7.77 ng/ml. This large concentration difference may be due to the inclusion of males, as B.E. H.I.P. females decreased their leptin concentration by 4.30 ng/ml. Park et al. [54], found no significant changes in adiponectin (mean increase of 0.3 μg/ml), while B.E. H.I.P. had a significant mean increase of 6.07 μg/ml in all participants and 5.14 μg/ml in females. Park et al. [54], hypothesized that their lack of significant changes might have been due to insufficient body fat changes, however as they were similar to the changes in B.E. H.I.P. participants, this cannot be the only explanation. They also suggested that the exercise programme may have been too modest in nature, therefore it could not promote adiponectin production and circulation [54]. It is hypothesized that the intensity and type of exercises performed in B.E. H.I.P., rather than simply the change in body composition, led to adiponectin and leptin changes.
Limitations
There are limitations to this study. Firstly, this study was based on convenience sampling. Although this methodology provided high adherence and a wealth of knowledge for future research, it cannot be generalized because the reference population is unknown. Nonetheless, the results are likely relevant to other 5 to 10 year old children who are overweight and\or obese and come from a similar background as children living in the Calgary, Alberta, Canada area. Secondly, socio-economic status was not controlled in this study. This was a potential confounder, but may not have been a significant factor in the study. This was hypothesized because children throughout the Calgary area participated in this study and it was not limited to one neighbourhood. There was no cost associated with this study, therefore families were not financially restricted and broader backgrounds were able to participate. Finally, no biological assessment was made for the pubertal status of the participants and parental information was assumed to be correct. Although age was not significantly correlated with adiponectin or leptin, sex was an important independent predictor of leptin. Future studies should determine the sex hormone concentrations to ascertain that the children are prepubescent.
Conclusion
The B.E. H.I.P. study demonstrated that a combined lifestyle and exercise intervention programme that progressively increased high intensity exercise and utilizes clinical biomarkers holds promise for childhood obesity prevention. A progressive increase of high intensity exercise is able to increased adiponectin and decreased leptin concentrations in overweight and obese children. It further highlighted that males and females responded differently to high intensity exercise, as sex was an important predictor of the leptin concentration change and for the duration of high intensity exercise. Continued research in the area of leptin, adiponectin and sex is required to further elucidate the potential role of these adipocytokines as biological markers in childhood obesity. High intensity exercise is a recommended tool for future prevention studies of childhood obesity.
Acknowledgements
We would like to extend our appreciation to Dr. Keith Sharkey for his knowledge and expertise and to Phil Landis and Tania Murynka, the exercise class instructors. Thank you to all the B.E. H.I.P. families and Tyler Williamson for his statistical knowledge. The Canadian Institute of Health Research-Institute of Gender and Health, supported this research.
References
- Shields M (2006) Overweight and obesity among children and youth. Health Rep 17: 27-42. [Crossref]
- Willms JD, Tremblay MS, Katzmarzyk PT (2003) Geographic and demographic variation in the prevalence of overweight Canadian children. Obes Res 11: 668-673. [Crossref]
- Fantuzzi G (2005) Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol 115: 911-919. [Crossref]
- Tilg H, Moschen AR (2006) Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 6: 772-783. [Crossref]
- Prentice RL, Willett WC, Greenwald P, Alberts D, Bernstein L, et al. (2004) Nutrition and physical activity and chronic disease prevention: research strategies and recommendations. J Natl Cancer Inst 96: 1276-1287. [Crossref]
- Venner AA, Lyon ME, Doyle-Baker PK (2006) Leptin: a potential biomarker for childhood obesity? Clin Biochem 39: 1047-1056. [Crossref]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, et al. (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425-432. [Crossref]
- Friedman JM, Halaas JL (1998) Leptin and the regulation of body weight in mammals. Nature 395: 763-770. [Crossref]
- Ahmed ML, Ong KK, Morrell DJ, Cox L, Drayer N, et al. (1999) Longitudinal study of leptin concentrations during puberty: sex differences and relationship to changes in body composition. J Clin Endocrinol Metab 84: 899-905. [Crossref]
- Arslanian S, Suprasongsin C, Kalhan SC, Drash AL, Brna R, et al. (1998) Plasma leptin in children: relationship to puberty, gender, body composition, insulin sensitivity, and energy expenditure. Metabolism 47: 309-312. [Crossref]
- Blum WF1, Englaro P, Hanitsch S, Juul A, Hertel NT, et al. (1997) Plasma leptin levels in healthy children and adolescents: dependence on body mass index, body fat mass, gender, pubertal stage, and testosterone. J Clin Endocrinol Metab 82: 2904-2910. [Crossref]
- Falorni A, Bini V, Molinari D, Papi F, Celi F, et al. (1997) Leptin serum levels in normal weight and obese children and adolescents: relationship with age, sex, pubertal development, body mass index and insulin. Int J Obes Relat Metab Disord 21: 881-890. [Crossref]
- Hassink SG, Sheslow DV, de Lancey E, Opentanova I, Considine RV, et al. (1996) Serum leptin in children with obesity: relationship to gender and development. Pediatrics 98: 201-203. [Crossref]
- Lahlou N, Landais P, De Boissieu D, Bougnères PF (1997) Circulating leptin in normal children and during the dynamic phase of juvenile obesity: relation to body fatness, energy metabolism, caloric intake, and sexual dimorphism. Diabetes 46: 989-993. [Crossref]
- Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, et al. (1995) Identification and expression cloning of a leptin receptor, OB-R. Cell 83: 1263-1271. [Crossref]
- Lammert A, Kiess W, Bottner A, Glasow A, Kratzsch J (2001) Soluble leptin receptor represents the main leptin binding activity in human blood. Biochem Biophys Res Commun 283: 982-988. [Crossref]
- Zastrow O1, Seidel B, Kiess W, Thiery J, Keller E, et al. (2003) The soluble leptin receptor is crucial for leptin action: evidence from clinical and experimental data. Int J Obes Relat Metab Disord 27: 1472-1478. [Crossref]
- van Dielen FM, van 't Veer C, Buurman WA, Greve JW (2002) Leptin and soluble leptin receptor levels in obese and weight-losing individuals. J Clin Endocrinol Metab 87: 1708-1716. [Crossref]
- Cinaz P, Bideci A, Camurdan MO, Güven A, Gönen S (2005) Leptin and soluble leptin receptor levels in obese children in fasting and satiety states. J Pediatr Endocrinol Metab 18: 303-307. [Crossref]
- Reinehr T, Kratzsch J, Kiess W, Andler W (2005) Circulating soluble leptin receptor, leptin, and insulin resistance before and after weight loss in obese children. Int J Obes (Lond) 29: 1230-1235. [Crossref]
- Hu E, Liang P, Spiegelman BM (1996) AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem 271: 10697-10703. [Crossref]
- Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, et al. (1996) cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1). Biochem Biophys Res Commun 221: 286-289. [Crossref]
- Nakano Y, Tobe T, Choi-Miura NH, Mazda T, Tomita M (1996) Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem 120: 803-812. [Crossref]
- Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF (1995) A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 270: 26746-26749. [Crossref]
- Arita Y1, Kihara S, Ouchi N, Takahashi M, Maeda K, et al. (1999) Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257: 79-83. [Crossref]
- Diamond FB Jr, Cuthbertson D, Hanna S, Eichler D (2004) Correlates of adiponectin and the leptin/adiponectin ratio in obese and non-obese children. J Pediatr Endocrinol Metab 17: 1069-1075. [Crossref]
- Martin LJ, Woo JG, Daniels SR, Goodman E, Dolan LM (2005) The relationships of adiponectin with insulin and lipids are strengthened with increasing adiposity. J Clin Endocrinol Metab 90: 4255-4259. [Crossref]
- Valle M, Martos R, Gascón F, Cañete R, Zafra MA, et al. (2005) Low-grade systemic inflammation, hypoadiponectinemia and a high concentration of leptin are present in very young obese children, and correlate with metabolic syndrome. Diabetes Metab 31: 55-62. [Crossref]
- Beltowski J (2003) Adiponectin and resistin--new hormones of white adipose tissue. Med Sci Monit 9: RA55-61. [Crossref]
- Gil-Campos M, Cañete R, Gil A (2004) Hormones regulating lipid metabolism and plasma lipids in childhood obesity. Int J Obes Relat Metab Disord 28 Suppl 3: S75-80. [Crossref]
- Stefan N, Stumvoll M (2002) Adiponectin--its role in metabolism and beyond. Horm Metab Res 34: 469-474. [Crossref]
- Yamauchi T, Hara K, Kubota N, Terauchi Y, Tobe K, et al. (2003) Dual roles of adiponectin/Acrp30 in vivo as an anti-diabetic and anti-atherogenic adipokine. Curr Drug Targets Immune Endocr Metabol Disord 3: 243-254. [Crossref]
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, et al. (2001) The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7: 941-946. [Crossref]
- Shand BI, Scott RS, Elder PA, George PM (2003) Plasma adiponectin in overweight, nondiabetic individuals with or without insulin resistance. Diabetes Obes Metab 5: 349-353. [Crossref]
- Reinehr T, Roth C, Menke T, Andler W (2004) Adiponectin before and after weight loss in obese children. J Clin Endocrinol Metab 89: 3790-3794. [Crossref]
- Lazzer S, Vermorel M, Montaurier C, Meyer M, Boirie Y (2005) Changes in adipocyte hormones and lipid oxidation associated with weight loss and regain in severely obese adolescents. Int J Obes (Lond) 29: 1184-1191. [Crossref]
- Doyle-Baker PK, Venner AA, Lyon ME, Fung T (2011) Impact of a combined diet and progressive exercise intervention for overweight and obese children: the B.E. H.I.P. study. Appl Physiol Nutr Metab 36: 515-525. [Crossref]
- (1998) American College of Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc 30: 975-991. [Crossref]
- Keskin M, Kurtoglu S, Kendirci M, Atabek ME, Yazici C (2005) Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics 115: e500-e503. [Crossref]
- Dupont WD (2002) Statistical Modeling for Biomedical Researchers: a Simple Introduction to the Analysis of Complex Data. Cambridge, Cambridge University Press, USA.
- Atlantis E, Barnes EH, Singh MA (2006) Efficacy of exercise for treating overweight in children and adolescents: a systematic review. Int J Obes (Lond) 30: 1027-1040. [Crossref]
- Gutin B (2008) Child obesity can be reduced with vigorous activity rather than restriction of energy intake. Obesity (Silver Spring) 16: 2193-2196. [Crossref]
- Wittmeier KD, Mollard RC, Kriellaars DJ (2008) Physical activity intensity and risk of overweight and adiposity in children. Obesity (Silver Spring) 16: 415-420. [Crossref]
- Mansoub S, Chan MK, Adeli K (2006) Gap analysis of pediatric reference intervals for risk biomarkers of cardiovascular disease and the metabolic syndrome. Clin Biochem 39: 569-587. [Crossref]
- Venner AA, Doyle-Baker PK, Lyon ME, Fung TS (2009) A meta-analysis of leptin reference ranges in the healthy paediatric prepubertal population. Ann Clin Biochem 46: 65-72. [Crossref]
- Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, et al. (1995) Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1: 1155-1161. [Crossref]
- Rosen CJ, Bouxsein ML (2006) Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol 2: 35-43. [Crossref]
- Rubin CT, Capilla E, Luu YK, Busa B, Crawford H, et al. (2007) Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc Natl Acad Sci U S A 104: 17879-17884. [Crossref]
- Huang L, Wang Z, Li C (2001) Modulation of circulating leptin levels by its soluble receptor. J Biol Chem 276: 6343-6349. [Crossref]
- Nemet D, Wang P, Funahashi T, Matsuzawa Y, Tanaka S, et al. (2003) Adipocytokines, body composition, and fitness in children. Pediatr Res 53: 148-152. [Crossref]
- Cambuli VM, Musiu MC, Incani M, Paderi M, Serpe R, et al. (2008) Assessment of adiponectin and leptin as biomarkers of positive metabolic outcomes after lifestyle intervention in overweight and obese children. J Clin Endocrinol Metab 93: 3051-3057. [Crossref]
- Matsubara M, Maruoka S, Katayose S (2002) Inverse relationship between plasma adiponectin and leptin concentrations in normal-weight and obese women. Eur J Endocrinol 147: 173-180. [Crossref]
- Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, et al. (2000) Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood 96: 1723-1732. [Crossref]
- Park TG, Hong HR, Lee J, Kang HS (2007) Lifestyle plus exercise intervention improves metabolic syndrome markers without change in adiponectin in obese girls. Ann Nutr Metab 51: 197-203. [Crossref]




