Abstract
Objectives
The brain’s inner cavities containing cerebrospinal fluid are referred to as ventricles, which are easily identified in an ultrasonographic (US) image as hypoecogenic and in computed tomography (CT) as hypodense. Imaging has become essential to planning and neuronavigation in brain surgeries, serving to guide the surgeon through the organ. The study aimed to 1) compare ultrasonography (US) and computed tomography (CT) measurements of lateral ventricle volumes (LVV) in dogs to determine their level of agreement and 2) understand their influence on surgical planning and neuronavigation.
Materials and methods
Measurements were taken from a sample of 24 LVVs from 12 brachycephalic dog cadavers. First, 2 mm slices of the dogs’ brains were scanned with the CT machine. The US brain scan technique was performed using a transducer of 7.5 MHz over the brain after craniotomy placement. This procedure was useful in simulating the brain neuronavigation technique in a two-dimensional scenario. A P value less than 0.05 was defined as statistically significant.
Results
A comparison of the US and CT images showing measurements of the right and left LVVs showed no statistically significant differences between the LVVs, allowing us to conclude that US is a reliable technique to planning and neuronavigation in brain surgeries.
Conclusion
As a result US is of great value in the everyday practice and areas working with limited resources such as in veterinary medicine, or even in a zooubitquity context, since in some human’s hospitals of developing countries or in some rural and remote areas, access to CT, magnetic resonance MR techniques, and neuronavigation systems are limited to point high-technology centers, unlike the sonography (US). Therefore, US it is a reliable technique to planning and neuronavigation in brain surgeries, thus promoting patient safety and surgeon success.
Key words
dog, lateral ventricle, ultrasonography, computed tomography, neuronavigation
Introduction
The brain’s inner cavities containing cerebrospinal fluid (CSF) are referred to as ventricles [1]. Four ventricles form the ventricular system: the right and left lateral ventricles, the third ventricle and the fourth ventricle [2-6]. The two largest ventricles are the right and left lateral ventricles, which are located in the cerebral hemispheres. These ventricles communicate across the middle-line level with the third ventricle (through the interventricular foramen or the foramina of Monro). Both have a C-shaped structure and are separated by the telencephalic midline septum, caudally diverging and ending at the piriform lobe. Each lateral ventricle presents three horns: an anterior or frontal horn extending to the frontal lobe, a posterior or occipital horn extending to the occipital lobe and an inferior or temporal horn extending into the temporal lobe. The floor of the lateral ventricles’ central part consists inferiorly of the caudate nucleus and posteriorly of the hippocampus [1,7]. The ventricular system is filled by the CSF, which is easily identified in an ultrasonographic (US) image as hypoecogenic and in computed tomography (CT) as hypodense [6,8-10]. Imaging has become essential to planning and neuronavigation in brain surgeries, serving to guide the surgeon through the organ [11-13]. Unlike US, access to CT and magnetic resonance (MR) techniques in veterinary medicine is limited to point centres. Compared with conventional neuroimaging methods, such as CT and MR, US is operator-dependent and have the advantages of low costs, short investigation times and repeatability; in addition, it does not have any harmful biological effects [14-17]. It is particularly suitable for imaging soft tissue and blood vessels. US is readily available, and it provides good images of brain tissue. It is a useful tool in surgical planning, and it can be used intraoperatively as a neuronavigation system to recognize brain structures [18-21]. Because of these advantages, it is important to exploit the benefits of this technique and integrate it into the surgical field, allowing its daily use in the medical and surgical fields to promote precise surgical work and reduce patient morbidity [22-25]. This study aimed to 1) compare US and CT measurements of lateral ventricle volumes (LVVs) in dogs to determine their levels of agreement and 2) understand their influence on surgical planning and neuronavigation.
Materials and methods
This study used a sample of 24 LVVs from 12 brachycephalic dog cadavers. The study protocol was approved by the Ethics Committee of Animal Welfare (CEBEA), Faculty of Veterinary Medicine, University of Lisbon – FMV/ULisboa, and Anjos of Assis Veterinary Medicine Centre (CMVAA), a veterinary surgeon provided clinical death verification and the owners signed consent forms. The age, body weight, and gender for all specimens were registered. After placing the heads in a prone position (to maintain symmetry during image acquisition), 2 mm-thick brain slices were scanned using a PHILIPS Mx8000 CT machine (Philips, Amsterdam, Netherlands). The US brain scans were performed with the Aloka ProSound SSD-3500 Plus and an electronic convex transducer from the intraoperative family (model UST-9104–5, Aloka, Zug, Switzerland) in B-mode real-time ultrasound with a frequency of 7.5 MHz. The US scan was performed after craniotomy placement, and the procedure was useful in simulating the brain neuronavigation technique in a two-dimensional scenario [26,27]. Transverse images of the brain were obtained by orienting the transducer on the brain surface and angling it in the anterior to posterior direction in a progressive manner. The process was repeated with the transducer angled mediolaterally to obtain sagittal images; the transducer was first oriented on the right hemisphere and then on the left hemisphere. After collecting the images, we measured the right and left LVVs in both image types (US and CT). To obtain LVV measurements in each specimen, the selected areas were delimitated using the “select” menu followed by the “measurements” command to obtain the LVV values. Statistical analysis was performed with Microsoft Excel (Microsoft Office 365) and the IBM SPSS Statistics (PASW Statistics 20, 2011) software program. To test the sample normality, we used the Shapiro-Wilk (W) test. The t-test for dependent means was used to analyse variations. To measure the strength of the linear relationship between two variables, we used the Pearson coefficient (r) and defined a P value less than 0.05 as statistically significant.
Results
All of the 24 lateral ventricles were easy to identify and evaluate as hypoecogenic structures using US and as hypodensity structures using CT. Table 1 shows the sample’s age, body weight, gender and breed characteristics as well as the LVV measurements for both hemispheres (right and left) using the US and CT techniques. The Shapiro-Wilk test supports the normality of the data for age (P = 0.85), body weight (P = 0.80) and measurements of the right and left LVVs obtained using US and CT (P = 0.85 for all). Using the US and CT images, it was possible to observe that the left lateral ventricle was always larger than the right lateral ventricle (Figure 1). Using the t-test for dependent means, it was possible to find statistically significant differences between age and the right and left LVVs in the US (P = 0.00 for both) and CT images (P = 0.00). The same results were found for body weight and the right and left LVVs in the US (P = 0.00 for both) and CT images (P = 0.00). No statistically significant differences were found for the measurements of the right LVV or the left LVV (P = 0.63) between the US and CT images (P = 0.34) (Table 2). Using the Pearson correlation it was possible to verify that the relationship was weak between the right LVV US and right LVV CT (r = 0.25); moderate between the right and left LVV CT (r = 0.50); and strong between the right and left LVV US (r = 0.67) and the left LVV US and left LVV CT (r = 0.79) (the maximum association value is one) (Table 2 and Figure 2).
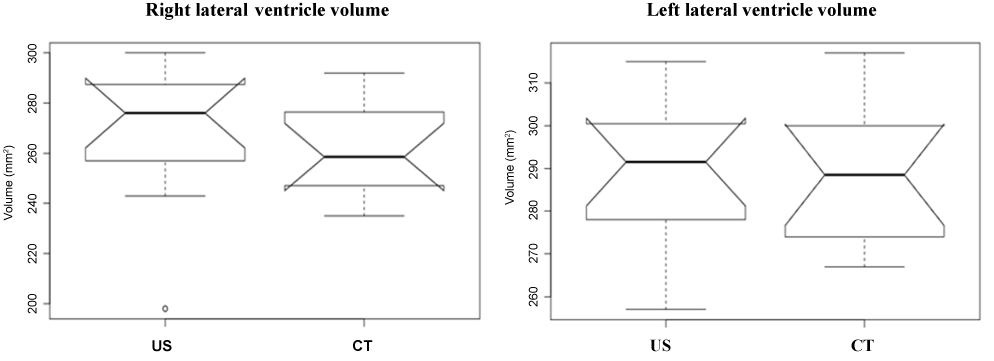
Figure 1. Comparison of left lateral ventricle volume with left lateral ventricle volume
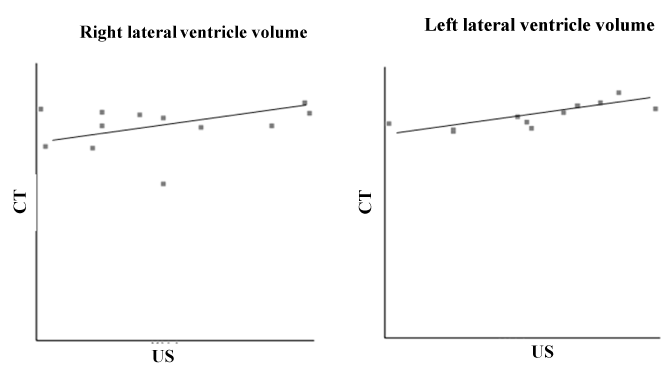
Figure 2. Pearson correlation of right and left ventricule volumes measured with ultra-sound
Table 1. Sample characterization for age, body weight, gender, breed, and lateral ventricles volumes (mm2) using the ultrasonography (US) and computed tomography (CT) images.
|
Parameter
|
|
Age
|
Body-weight
|
Gender
|
Breed
|
n
|
|
± SD
|
min
|
max
|
± SD
|
min
|
max
|
Female
|
Male
|
|
8.66 ± 2.30
|
5
|
14
|
3.30 ± 1.58
|
2.04
|
6.7
|
41.6
|
58.4
|
Total sample
|
12
|
|
9.33 ± 2.17
|
7
|
14
|
2.47 ± 0.30
|
2.04
|
3.01
|
37.5
|
62.5
|
Chihuahua
|
8
|
|
7.5 ± 0.70
|
7.0
|
8.0
|
5.78 ± 1.29
|
4.87
|
6.7
|
50.0
|
50.0
|
Pequinois
|
3
|
|
5.0
|
-
|
-
|
5.87
|
-
|
-
|
-
|
100
|
Shi-tzu
|
1
|
|
Ultrasound
|
Computed Tomography
|
|
Right LVV (mm2)
|
Left LVV(mm2)
|
Right LVV (mm2)
|
Left LVV (mm2)
|
|
± SD
|
min
|
max
|
± SD
|
min
|
max
|
± SD
|
min
|
max
|
± SD
|
min
|
max
|
|
269.17 ± 28.40
|
198
|
300
|
289.58 ± 16.74
|
257
|
315
|
260.58 ± 19.86
|
235
|
292
|
288.08 ± 15.82
|
267
|
317
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
Mean (); standard deviation (SD); minimum (min); maximum (max); sample (n); lateral ventricle volume (LVV)
Table 2. Sample t-test dependent means and the Pearson correlations (r) for analysis the right and left ventricle volumes measured with ultra-sound (US) and computerized tomography (TC). Significant values for p<0.05.
|
Type of test
|
Parameter
|
Comparison
between group
|
n
|
Differences
between means
|
p-value
|
r
|
|
t-test dependent means
|
Age
|
RLVV US
|
12
|
260.5
|
0.00
|
-
|
|
RLVV TC
|
12
|
251.92
|
0.00
|
-
|
|
LLVV US
|
12
|
280.92
|
0.00
|
-
|
|
LLVV TC
|
12
|
279.42
|
0.00
|
-
|
|
Body-weight
|
RLVV US
|
12
|
265.86
|
0.00
|
-
|
|
RLVV TC
|
12
|
257.28
|
0.00
|
-
|
|
LLVV US
|
12
|
286.28
|
0.00
|
-
|
|
LLVV TC
|
12
|
284.78
|
0.00
|
-
|
|
Ventricle volume and Technique
|
RLVV US / RLVV TC
|
24
|
8.58
|
0.34
|
-
|
|
LLVV US / LLVV TC
|
24
|
-1.5
|
0.63
|
-
|
|
Pearson Correlation
|
Ventricle volume and Technique
|
RLVV US / RLVV TC
|
24
|
-
|
-
|
0.25
|
|
RLVV US / LLVVVUS
|
24
|
-
|
-
|
0.67
|
|
LLVV US / LLVV TC
|
24
|
-
|
-
|
0.79
|
|
RLVV TC / LLVV TC
|
24
|
-
|
-
|
0.50
|
Sample (n); right lateral ventricle volume (RLVV); left lateral ventricle volume (LLVV)
Discussion
Technologically advanced imaging techniques, such as US, CT and MR, have revolutionized brain medicine and surgery, enabling easier access to the organ and thus improving diagnostic skills and the implementation of less invasive techniques [26,28,29]. The use of a convex transducer with a frequency of 7.5 MHz performs accurate brain scans, thus allowing the identification of different brain structures and detailed neuronavigation. In this study, we performed the US brain scan technique after craniotomy placement to acquire brain images and simulate the brain neuronavigation technique in a two-dimensional scenario as described by Unsgaard et al. [27] We became identified images of different brain structures that can act as landmarks during surgical procedures. The right and left lateral ventricles have a hypoecogenic pattern and are located bilaterally in an inferior plane of the cerebral falx. These hypoecogenic structures were easily visualized in the transverse and sagittal planes in all the brain [30-32]. The shape of the lateral ventricular system was flatter and broader in all specimens. The hypoecogenic pattern occurs because the CSF that fills these structures is synthesized by the choroid plexus, [33] which was not always easy to identify in the US and CT images. The choroid plexus corresponds to capillary tufts lined by a simple cylindrical epithelial layer originating in the ependyma, which makes them highly reflective with a US hyperechoic pattern [30,32,34,35]. Because this study used cadavers, no circulation was present. This may explain why, in the US image, the choroid plexus was easy to identify in only 13% of the specimens. Other structures directly related to the lateral ventricles were also evaluated with the US images, including the dorsal portion of the caudate nucleus (a hyperecogenic structure that was easy to identify in 4.4% of the samples, moderately difficult to identify in 73.9% of the samples and difficult to identify in 21.7% of the samples) and the hippocampus (a hypoecogenic structure that easy to identify in 91.3% of the samples, moderately difficult to identify in 4.4% of the samples and difficult to identify in 4.3% of the samples). In addition, the dura mater, cerebral falx and pia mater were easy to visualize, and they all showed a hyperecogenic pattern. Distinguishing other brain structures was not easy in these specimens, which might be related to the cranial geometry of the breed (brachycephalic). Inducing a brain adaptation to the vault architecture required compression in the dorsoventral axis, thus increasing the difficulty of identifying different brain regional structures. According to Schroeder et al., [36] brachycephalic breeds have larger LVVs than dolichocephalic and mesocephalic breeds [36-41]. Based on our results, age appears to influence the LVV in a statistically significant way, which Sue et al. also found [42]. However, this correlation is not linear because the ventricular volume gradually increases until the age of 10, after which the increase is much more pronounced. This pronounced increase is associated with brain aging atrophy, which induces ventricular system enlargement [43-47]. In addition, significant differences were seen between the specimen’s body weight and the LVV, as reported by Vite et al. [38]. Vite et al. [38] found that individuals with lower body weight percentages had higher LVV values. No statistically significant differences were seen between the LVV measurement values obtained using the US and CT techniques in this study. However, asymmetries between both LVVs were found, with the left lateral ventricle always larger than the right lateral ventricle. The asymmetry of the lateral ventricles is considered normal in healthy dogs up to a certain value [36,37,39,48,49]. In a study using Labrador retrievers, De Haan et al. [37] estimated an asymmetry of 31%. This is similar to the results in Winchester et al. [50] which used US to confirm the presence of asymmetry in the lateral ventricles in humans. In our study, we verified the lateral ventricular asymmetry, with a predominant increase in the left LVV in 75% of all cases using US and an increase in 91.7% of all cases using CT. The value obtained with US is quite similar to that referred to in Schröder et al. [36] which found that the left LVV in 67% of all cases was wider than the right LVV. The differences between LVV measurements obtained using US and CT could be because a craniectomy was performed to access the brain when acquiring US images, thus inducing changes in the intracranial anatomy and, therefore, bias in the measurements [14,15]. In addition, the LVV measurements obtained using US could change depending on the angle of the probe over the brain surface, thus allowing image collection but not corrections, as is possible with the CT technique [50].
Concluding remarks
Nevertheless, the US technique has shown excellent agreement with the measurements obtained by CT scans, with no statistically significant differences. As a result US is of great value in the everyday practice and areas working with limited resources such as in veterinary medicine, or even in a zooubitquity context, since in some human’s hospitals of developing countries or in some rural and remote areas, access to CT, magnetic resonance MR techniques, and neuronavigation systems are limited to point high-technology centers, unlike the sonography (US). Therefore, US it is a reliable technique to planning and neuronavigation in brain surgeries, thus promoting patient safety and surgeon success.
Acknowledgments
The authors thank to Center for Interdisciplinary Research in Animal Health – CIISA – FVM/ULisboa; to Prof. Antonio Ferreira, Profª Sandra de Jesus, and Dr. Oscar Gamboa from FMV/ULisboa; and to Anjos of Assis Veterinary Medicine Centre (CMVAA), Barreiro – Portugal.
References
- Dellmann HD, Mc Clure RG (1986) Sistema Nervoso Central. In: Getty, Sisson, Grossman’s eds. A Anatomia Dos Animais Domésticos. 5th ed, Editora Guanabara Koogan SA, Rio de Janeiro,186-206.
- Dyce K, Sack W, Wensing C (1996) Textbook of Veterinary Anatomy. Philadelphia, USA:W.B.Saunders.
- Colaço B, Ferreira D, Gonzalo-Ordén M, Villar Lacilla J (2003) A aplicação da ressonância magnética no estudo anatómico do encéfalo de cães The use of magnetic resonance imaging in the study of canine brain anatomy. Rev Port Ciências Vet 98: 159-165.
- Osborn AG, Blaser S, Slazman K, Katzman GL, Provenzale J, Castillo M, Hedlund GL, Illner A, Hansberger HR, Cooper J, Jones B, Hamilton B (2004) Diagnostic imaging brain. Amirsys-A medical reference publishing company, (1st Edn.), Altona, Manitoba, Canada, Printed by Friesens.
- Reece WO (2004) Functional Anatomy and Physiology of Domestic Animals. (3rd Edn.), Wiley, John & Sons, Incorporated, 146-149.
- Igbaseimokumo U (2009) Brain CT Scans in Clinical Practice. London: C Springer-Verlag.
- Kim JH, Jeon HW, Woo EJ, Park HM (2009) Dilation of the olfactory bulb cavity concurrent with hydrocephalus in four small breed dogs. J Vet Sci 10: 173-175. [Crossref]
- Cummings BJ, Head E, Ruehl W, Milgram NW, Cotman CW (1996) The canine as an animal model of human aging and dementia. Neurobiol Aging 17: 259-268.[Crossref]
- Lucas RAP, Godoy RC, Sacco SR (2008) Análise do Líquido Cefalorraquidiano em Pequenos Animais. Revista Cientifica Eletrónica de Medicina Veterinária 11: 1-7.
- Kapoor KG, Katz SE, Grzybowski DM, Lubow M (2008) Cerebrospinal fluid outflow: an evolving perspective. Brain Res Bull 77: 327-334. [Crossref]
- Chesnut RM (1998) Implications of the guidelines for the management of severe head injury for the practicing neurosurgeon. Surg Neurol 50: 187-193. [Crossref]
- Chesnut RM, Marshall LF, Klauber MR, Blunt BA, Baldwin N, et al. (1993) The role of secondary brain injury in determining outcome from severe head injury. J Trauma 34: 216-222. [Crossref]
- Bullock R, Chesnut RM, Clifton G, Ghajar J, Marion DW, et al. (1996) Guidelines for the management of severe head injury. Brain Trauma Foundation. Eur J Emerg Med 3: 109-127. [Crossref]
- Caricato A, Pitoni S, Montini L, Bocci MG, Annetta P, et al. (2014) Echography in brain imaging in intensive care unit: State of the art. World J Radiol 6: 636-642. [Crossref]
- Caricato A, Mignani V, Bocci MG, Pennisi MA, Sandroni C, et al. (2012) Usefulness of transcranial echography in patients with decompressive craniectomy: a comparison with computed tomography scan. Crit Care Med 40: 1745-1752. [Crossref]
- Godani1 M, Francesca Canavese, Massimo Del Sette, Uwe W (2014) Update on Transcranial Sonography Applications in Movement Disorders. J Diagnostic Imaging in Therapy 1: 110-128.
- Libert N, Boutonnet M, Giraud N, Tourtier JP, de Rudnicki S (2012) Transcranial echography: an interesting tool for aeromedical evacuations. Crit Care Med 40: 3331-3332. [Crossref]
- Comeau RM, Fenster A, Peters TM (1998) Intraoperative US in interactive image-guided neurosurgery. Radiographics 18: 1019-1027. [Crossref]
- Tuominen J, Yrjänä SK, Katisko JP, Heikkilä J, Koivukangas J (2003) Intraoperative imaging in a comprehensive neuronavigation environment for minimally invasive brain tumour surgery. Acta Neurochir Suppl 85: 115-120. [Crossref]
- Lunn KE, Paulsen KD, Roberts DW, Kennedy FE, Hartov A, et al. (2003) Displacement estimation with co-registered ultrasound for image guided neurosurgery: a quantitative in vivo porcine study. IEEE Trans Med Imaging 22: 1358-1368. [Crossref]
- Jödicke A, Springer T, Böker DK (2004) Real-time integration of ultrasound into neuronavigation: technical accuracy using a light-emitting-diode-based navigation system. Acta Neurochir (Wien) 146: 1211-1220. [Crossref]
- Seeger W (1980) Microsurgery of the Brain, Anatomical and Technical Principles. Vienna: Springer.
- Horsley V (1986) On the topographical relations of the cranium and the surface of the cerebrum. In: Cunningham, Horsley V: On the topographical relations of the Cranial Contribution to the Surface Anatomy of the Cerebral Hemispheres. Dublin: Academy House, 306-355.
- Ono M, Kubik S, Abernathey C. Atlas of cerebral sulci. New York, Stuttgart: Thieme-Verlag, 1990
- Rhoton AJ. Cranial anatomy and surgical approaches. Neurosurgery 2003;53(1):746.
- Brown FD, Rachlin JR, Rubin JM, Fessler RG, Smith LJ, et al. (1984) Ultrasound-guided periventricular stereotaxis. Neurosurgery 15: 162-164. [Crossref]
- Unsgaard G, Gronningsaeter A, Ommedal S, Nagelhus Hernes TA. Brain operations guided by real-time 2D ultrasound - New possibilities due to improved image quality. Neurosurgery 2003
- Gallagher JG, Penninck D, Boudrieau RJ, Schelling SH, Berg J (1995) Ultrasonography of the brain and vertebral canal in dogs and cats: 15 cases (1988-1993). J Am Vet Med Assoc 207: 1320-1324. [Crossref]
- Tucker RL, Gavin PR (1996) Brain imag2021 Copyright OAT. All rights reservct 26: 735-758. [Crossref]
- Carvalho C. Ultra-sonografia em Pequenos Animais. São Paulo, Brazil: Roca, 2004
- Angtuaco TL (2005) Ultrasound imaging of fetal brain abnormalities: three essential anatomical levels. Ultrasound Q 21: 287-294. [Crossref]
- Ichihashi K, Yada Y, Takahashi N, Honma Y, Momoi M. Analysis of the intensity of radio-frequency signals in intracranial ultrasonography of preterm infants. J Med Ultrasonics 2008;35(2):1346-4523.
- Lahunta A (1983) Cerebellum. In: A. de Lahunta (Eds.), Veterinary Neuroanatomy and Clinical Neurology. Philadelphia, USA, W.B. Saunders Co, pp: 255-278.
- Fiske CE, Filly RA, Callen PW (1981) The normal choroid plexus: ultrasonographic appearance of the neonatal head. Radiology 141: 467-471. [Crossref]
- Tipold A (2003) Cerebrospinal fluid. In: Clinical Neurology in Small Animals –Localization, diagnosis and treatment. K.G. Braund Ed.,International Veterinary Service, Ithaca, New York, USA.
- Schröder H, Meyer-Lindenberg A, Nolte I (2006) Comparative examination of the lateral cerebral ventricles of different dog breeds using quantitative computed tomography. Berl Munch Tierarztl Wochenschr 119: 506-511.
- De Haan C, Kraft S, Gavin P, Wendling L, Griebnow M (1994) Normal variation in size of the lateral ventricles of the Labrador Retriever dog as assessed by magnetic resonance imaging. Vet Radiol Ultrasound 35: 83-86.
- Vite CH, Insko EK, Schotland HM, Panckeri K, Hendricks JC (1997) Quantification of cerebral ventricular volume in English bulldogs. Vet Radiol Ultrasound 38: 437-443. [Crossref]
- Kii S, Uzuka Y, Taura Y, Nakaichi M, Inokuma H, et al. (1998) Developmental change of lateral ventricular volume and ratio in Beagle-type dogs up to 7 months of age. Vet Radiol Ultrasound 39: 185-189. [Crossref]
- Esteve-Ratsch B, Kneissl S, Gabler C (2001) Comparative evaluation of the ventricles in the Yorkshire Terrier and the German Shepherd dog using low-field MRI. Vet Radiol Ultrasound 42: 410-413. [Crossref]
- Carreira LM1,2,3, Ferreira A1,2 (2015) Longitudinal Cerebral Fissure Anatomy Variations in Brachy-, Dolicho- and Mesaticephalic Dogs and Their Importance to Brain Surgery. Anat Rec (Hoboken) 298: 1612-1621. [Crossref]
- Su MY, Head E, Brooks WM, Wang Z, Muggenburg BA, et al. (1998) Magnetic resonance imaging of anatomic and vascular characteristics in a canine model of human aging. Neurobiol Aging 19: 479-485. [Crossref]
- Kamiya S, Yamagami T, Fujii S, Yamano S, Umeda M, et al. (1995) Senil Plaques in the Beagle Brain. Bull. Nippon Vet. Anim. Sci. Univ 44: 1-4.
- Kiatipattanasakul W, Nakamura S, Hossain MM, Nakayama H, Uchino T, et al. (1996) Apoptosis in the aged dog brain. Acta Neuropathol 92: 242-248. [Crossref]
- Borràs D, Ferrer I, Pumarola M (1999) Age-related changes in the brain of the dog. Vet Pathol 36: 202-211. [Crossref]
- González-Soriano J, Marín García P, Contreras-Rodríguez J, Martínez-Sainz P, Rodríguez-Veiga E (2001) Age-related changes in the ventricular system of the dog brain. Ann Anat 183: 283-291. [Crossref]
- Ingram DK, Williams N (2002) Neurobiology of Cognitive Dysfunction Syndrome in Dogs. Clinical and Nutritional Management of Senior Dogs and Cats. WSAVA, pp: 31-36
- Hudson J, Simpson S, Buxton D, Cartee R, Steiss J (1990) Ultrasonographic diagnosis of canine hydrocephalus. Vet Radiol. 31: 50-58.
- Spaulding KA, Sharp NJH (1990) Ultrasonographic imaging of the lateral cerebral ventricles in the dog. Vet Radiol Ultrasound 31: 59-64.
- Winchester P, Brill PW, Cooper R, Krauss AN, Peterson HD (1986) Prevalence of "compressed" and asymmetric lateral ventricles in healthy full-term neonates: sonographic study. AJR Am J Roentgenol 146: 471-475. [Crossref]


