Abstract
A 37-year-old patient with Complex Regional Pain Syndrome(CRPS) and continuous intrathecal morphine administration presents to prenatal care pregnant with 16 weeks’ gestation. Besides CRPS, she has other medical conditions like obesity, migraine and angina, using topiramate and diltiazem.
The discussion goes through an overview of CRPS and chronic pain management in pregnancy. In the end, we include a summary and recommendations based on a brief review of the literature.
Key words
complex regional pain syndrome, reflex sympathetic dystrophy, pregnancy, chronic pain management, chronic pain, opioids
Introduction
Obstetrics is a somewhat tricky part of Medicine, since every disease have its nuances in a pregnant woman and her fetus. So, it is with Complex Regional Pain Syndrome (CRPS), an uncommon illness that “describes an array of painful conditions that are characterized by a continuing (spontaneous and/or evoked) regional pain that is seemingly disproportionate in time and degree to the usual course of any known trauma or other lesion. The pain is regional (not in a specific nerve territory or dermatome) and usually has a distal predominance of abnormal sensory, motor, sudomotor, vasomotor and/or trophic findings” [1].
This condition itself doesn’t pose a threat to pregnancy, but its chronicle treatment may be of concern. The management usually involves opioids and non-steroidal anti-inflammatories, but new treatments are rising. Another question to consider is if medical growing “instrumentalization” of labor may cause CRPS and if there’s a way to prevent it.
Our goal is to raise awareness of this condition, bringing some practical piece of advice. We will include a brief discussion about chronic pain management in pregnancy. Lastly, we will leave a summary and recommendations.
Case report
A 37-year-old patient presented to us with a history of CRPS for the past 20 years in the wrist, after a volleyball injury. She tried different medications and pain-killers, including subcutaneous infusion-pump morphine and a cervical sympathectomy, and is actually using an intrathecal catheter for continuous morphine administration (Figure 1).
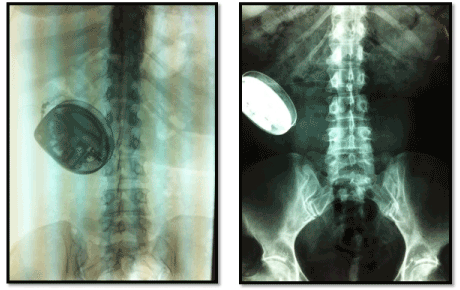
Figure 1. Radiographs showing the intrathecal morphine pump.
She experimented menstrual irregularity for 3 years and could not get pregnant despite the use of bromocriptin, giving up treatment. Surprisingly, she became pregnant spontaneously and discovered it around 16 weeks of gestation. Besides the CRPS, other medic conditions included obesity, sporadical migraine (topiramate) and vasospastic angina (diltiazem).
We decided to keep the intrathecal morphine infusion for pain control, slowly increasing the doses (Table 1), together with an intense prenatal care with close follow up. At 39 weeks, a cesarean section was performed as requested by the patient, without complications, and with a multidisciplinary team support. The patient recovered very well from the surgery. The newborn was at first observed for side effects in the ICU, but home discharged with her mother after two days at the hospital.
Gestational age |
Morphine dosing regimen |
1st trimester |
2.3mg/day |
2nd trimester |
2.5mg/day |
3rd trimester |
2.7mg/day |
Table 1. Comparing gestational age and morphine regimen during pregnancy with CRPS.
Discussion
As stated before, the discussion will involve an overview of chronic pain management in pregnancy and Complex Regional Pain Syndrome.
Pregnancy and chronic pain management
Coluzzi et al. [2] concludes that there are no specific and safe medications for pain management in pregnancy. There are six major options for chronic pain management: (1) pharmacologic, (2) physical medicine, (3) behavioral medicine, (4) neuromodulation, (5) interventional and (6) surgical approaches. Normally, optimal pain control is achieved by using multiple options instead of a single option treatment [3]. On top of that, chronic pain patients need continuous attention, reassurance and reevaluations. Pharmacologic treatment is the most common approach and has the widest array of agents, including many different pharmacological classes with different metabolic pathways which proportionate a great number of possible combinations in order to optimize pain control while diminishing unwanted collateral effects.
Chronic pain is divided in two groups with different etiologies. Neuropathic pain results from lesions in the peripheral or central nervous system, and includes a variety of causes like metabolic (eg. Diabetes Mellitus), infectious (eg. Herpes) and trauma (eg. Surgical nerve lesions) and commonly does not have obvious stimuli. Nociceptive pain, in the other hand, is caused by tissue damage or stimuli of pain receptors, but not necessarily involves nervous damage. Causes of nociceptive pain includes inflammation, mechanical/compressive problems etc.
Treatment of neuropathic pain often involves antidepressants (amitriptyline, nortriptyline, duloxetine, venlafaxine) or calcium channel ligands (gabapentin, pregabalin) [4,5], opioids being a second-line option (although some advocate its use in selected special cases [6]). All of them, are FDA pregnancy risk factor category “C” (Table 2), which means there’s no positive evidence of risk, but risk cannot be ruled out and drugs should only be given if the potential benefits justify the potential risk to the fetus.
Category |
Interpretation |
A |
Controlled studies in pregnant women fail to demonstrate an increased risk of fetal abnormalities to the fetus in the first trimester (and there is no evidence of a risk in later trimesters), and the possibility of fetal harm appears remote. |
B |
Either animal-reproduction studies not demonstrated a fetal risk, but there are no controlled studies in pregnant women, or animal-reproduction studies have shown as adverse effect (other than a decrease in fertility) that was not confirmed in controlled studies in women in the first trimester (and there is no evidence of a risk in later trimesters). |
C |
Either studies in animals have revealed adverse effects on the fetus (teratogenic or embriocydal or other) and there are no controlled studies in women, or studies in women and animals are not available. Drugs should be given only if the potential benefit justifies the potential risk to the fetus. |
D |
There is positive evidence of human fetal risk, but the benefits from use in pregnant women may be acceptable despite the risk (e.g. If the drug is needed in a life-threatening situation or for a serious disease for which safer drugs cannot be used or are ineffective). |
X |
Studies in animals or human beings have demonstrated fetal abnormalities, or there is evidence of fetal risk based on human experience or both, and the risk f the use of the drug in the pregnant women clearly outweighs any possible benefit. The drug is contraindicated in women who are or may become pregnant. |
Table 2. FDA medication categories in pregnancy.
Nocioceptive pain is treated mainly with nonnarcotic and opioid analgesia, and a special role is given to no pharmacologic therapies and approaches in order to relieve the source of the pain. Usual treatment approach starts with Acetaminophen/Paracetamol or Dipirone and combined with others medications, like NSAIDs and COX-2 inhibitors, and opioids, in this order. We discuss each kind of drug and its relation to pregnancy further in the topics below. The following recommendations are largely based on the work of Coluzzi et al. [2].
Non-steroidal anti-inflammatory drugs (NSAIDs): NSAIDs are the most common analgesics prescribed medication, and all of them have side effects on both mother and fetus. They should be avoided by women trying to conceive because some studies show deleterious effects on blastocyst implantation in animal models. Some NSAIDs (indomethacin, ibuprofen, naproxen) are considered safe to use for a short period of time before the 32nd week, since they do not seem to increase fetal malformation risks. But should be avoided in late pregnancy due to premature antenatal closure/narrowing of ductus arteriosus, leading to oligohydramnios and neonatal pulmonary hypertension.
Paracetamol (Acetaminophen) and Dipyrone: Data linking paracetamol to poor pregnancy and/or late childhood outcomes are conflicting, and is wise to avoid excessive or unnecessary use during pregnancy. Nevertheless, the maximum dose is 1g per day, and any dose higher will increase risks and side effects without increasing analgesic activity.
There is a lack of studies about dipyrone intake and its effects on pregnancy outcomes. A large Brazilian cohort, where dipyrone is a very common analgesic, found no data suggesting that the exposure during pregnancy “increased the risk of congenital abnormalities and other adverse events as outcomes from pregnancy”. Anecdotally, we used to prescribe Dipyrone for pain following fetal surgery, but we noticed an increased number of oligohydramnios. After its withdrawal from the routine, the incidence of oligohydramnios fell considerably. In a nutshell, use of dipyrone, as paracetamol, should be avoided unnecessarily.
Opioids: Opioids are known as potent painkillers and side effects, as well as its physical and psychological dependence. Common opioids in our practice include morphine, codeine, tramadol, fentanyl, methadone and others. All of them, because of their chemical characteristics, are able to crosse the placenta and blood-brain barrier, being encountered also in the breast-milk. Abstinence syndrome is well described, and happens with the abrupt discontinuation of medication, like after umbilical cord clamping.
Another concern is if opioids use pose a risk to birth defects. Literature is conflicting. Broussard et al. [7] case-control study found that therapeutic maternal opioid treatment within 1 month before and 3 months after conception was significantly associated with some types of congenital heart defects and, at a lower risk, other birth defects. Another study led by Yazdy et al. [8] reported an increased risk of neural tube defects. In the other hand, a large population-based cohort study in Norway comparing women who had used codeine during pregnancy with those who had not found no significant association between codeine use and survival, congenital malformation or respiratory depression [9].
Some patients need intolerable high doses of opioids in order to achieve pain control. These patients are candidates to intrathecal therapy, a system that delivers opioids directly to the opioids receptors in the spinal cord, thus minimizing its systemic effects. There are still no randomized trials evaluating the effectiveness of this method for chronic pain [10], and still no studies about its effect on pregnancy. In our case, the newborn had no signals of opioids dependence and/or neonatal abstinence syndrome, common in oral opioids. Complications differ, and some patients refers mostly nausea/vomiting and pruritus [11].
Antidepressants: Antidepressants are generally safe to use during pregnancy. Although some of them are classified as “D” by FDA (Table 2), Amitriptyline and Clomipramine are especially safe [12]. Usually, the American College of Obstetricians and Gynecologists and the American Psychiatric Association endorse the maintenance of antidepressants during pregnancy.
Antiepiletic drugs (AEDs): Studies around antiepiletic drugs safety are still inconclusive, since most studies exclude patients potentially pregnant. But besides this, pregnancy can affect the pharmacokinetics of AEDs in all levels, greatly increasing the risk of unexpected outcomes. Because of this, they should be used unless other medication for pain control are unavailable. Lamotrigine is the only AED considered safe, while others still need more data.
Corticosteroid: A recent Danish cohort with more than 800,000 live births showed no link between glucocorticoids use and poor pregnancy outcome. Instead, corticosteroids are being used in recurrent miscarriage with important improvement of fetal and maternal prognosis. Despite these findings, is a good practice to limit corticosteroids administration to the lowest effective dose.
Complex Regional Pain Syndrome (CRPS)
CRPS is a painful and severe disorder which affects a region of the body, usually distal limbs, and not rarely causes grave disabilities. It is characterized by chronic pain with autonomic symptoms, like swelling, vasomotor and sudomotor instabilities, and, in severe cases with great disability and movement impairments, patchy bone demineralization. Frequently appears after a trauma or minor injuries, since fractures to surgeries.
2021 Copyright OAT. All rights reserv
There are two types of CRPS [1]. Type 1, which represents 90% of cases, is also known as Reflex Sympathetic Dystrophy and presents without evidence of peripheral nerve injury. Type 2 was formerly called Causalgia, and presents with peripheral nerve injury.
Pathogenesis is unknown. Most theories propose some classical and normal noxious stimuli by inflammation, but defective central nervous response [13]. Collectively, the evidence points to CRPS being a multifactorial disorder that is associated with an aberrant host response to tissue injury [14]. Incidence is more common in women by two to four-fold rates. Fractures, sprains and crush injuries are the leading causes of CRPS, although surgeries and other traumas are related [15].
Initial symptoms usually appear after four to six weeks of the insult, and involves pain, erythema and swelling of the affected region [14]. As expected, diagnosis is based upon clinical presentation, history and physical examination, especially if the initial trauma no longer explains the symptoms. There no methods for confirming the diagnosis, but there are criteria that can be useful (Budapest Criteria) [1].
The best treatment for CRPS is prevention. Some studies associated vitamin C intake (500 mg/day) after a limb trauma/surgery with a significant reduction in the development of CRPS, when compared to placebo [16], suggesting a dose of 500 mg daily for 50 days. Treatment and management typically involves medication for neuropathic pain (refer to topic above about chronic pain management and pregnancy). During pregnancy and periconceptional phase, risks and benefits of each method must be discussed with the patient.
Prognosis of CRPS is uncertain, but a significantly share of patients evolves with some kind of chronic disability. A Dutch study [17] followed 102 patients for 5.8 years and reported that about 64% were still suffering with CRPS symptoms, while 30% considered themselves as recovered, 54% stable and 16% worse due to progressive disease. Only 41% could resume their work completely, and 31% were unable to work.
Estimated recurrence can occur between 10 to 30%, especially among younger patients, including children [18]. Risk factors observed to trigger the disease include cold exposure, new trauma/surgery or even spontaneously, and can affect the same limb or other regions.
Conclusion
Chronic pain management during pregnancy still pose a challenge, especially with refractory pain. Drugs normally used for pain control do have side effects and risks that must be discussed with the patient. Wise drug prescription avoids unnecessary analgesics administration and prefers the lowest dose necessary and for the shortest period of time.
We believe more studies and protocols are needed for safe pain management during pregnancy regarding maternal and fetal health, reducing risks of poor outcomes. Until there, we recommend the following:
- Periconceptional phase: avoid NSAIDs.
- First trimester: Paracetamol and Dipyrone are safe; avoid NSAIDs; Avoid opioids.
- Second trimester: Paracetamol and Dipyrone are safe; avoid NSAIDs; Opioids are safe.
- Third trimester: Do not use NSAIDs after 32 weeks’ gestation; avoid Dipyrone as routine. Opioids are safe, but neonatology should investigate neonatal abstinence syndrome.
- Antidepressants and AEDs can be continued with taken previously, but should not be introduced during pregnancy.
- Corticosteroids are safe to use during pregnancy, but its use is limited to conditions which benefits from steroidal anti-inflammatory treatment.
CRPS still is a neglected and poor diagnosed condition that greatly affects quality of life. The best approach still is prevention. Although rare, gynecological/obstetric surgery may evolve to CRPS. Despite this, we strongly believe there is no need for routine vitamin C intake for prevention, unless distal limbs are affected by a trauma or a surgery.
Based on our experience, intrathecal catheter opioid administration seems a promising method with great pain control and safety, reducing systemic effects and probably diminishing risks of fetal malformation and abstinence syndrome. In the other hand, there are few studies about this option, and still less during pregnancy.
References
- Harden RN, Bruehl S, Stanton-Hicks M, Wilson PR (2007) Proposed new diagnostic criteria for complex regional pain syndrome. Pain Med 8: 326-331. [Crossref]
- Coluzzi F, Valensise H, Sacco M, Allegri M (2014) Chronic pain management in pregnancy and lactation. Minerva Anestesiol 80: 211-224. [Crossref]
- Kamper SJ, Apeldoorn AT, Chiarotto A, Smeets RJ, Ostelo RW, et al. “Multidisciplinary biopsy hospital rehabilitation for chronic low back pain: Cochrane systematic review and meta-analysis”.
- Gilron I, Baron R, Jensen T (2015) Neuropathic pain: principles of diagnosis and treatment. Mayo Clin Proc 90: 532-545. [Crossref]
- Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, et al. (2015) Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 14: 162-173. [Crossref]
- Dworkin RH, O'Connor AB, Backonja M, Farrar JT, Finnerup NB, et al. (2007) Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain 132: 237-251. [Crossref]
- Broussard CS, Rasmussen SA, Reefhuis J, Friedman JM, Jann MW, et al. (2011) Maternal treatment with opioid analgesics and risk for birth defects. Am J Obstet Gynecol 204: 314. [Crossref]
- Yazdy MM, Mitchell AA, Tinker SC, Parker SE, Werler MM (2013) Periconceptional use of opioids and the risk of neural tube defects. Obstet Gynecol 122: 838-844. [Crossref]
- Nezvalová-Henriksen K, Spigset O, Nordeng H (2011) Effects of codeine on pregnancy outcome: results from a large population-based cohort study. Eur J Clin Pharmacol 67: 1253-1261. [Crossref]
- Harden RN, Argoff CE, Williams DA (2014) Intrathecal opioids for chronic pain: a call for evidence. Pain Med 15: 1823-1824. [Crossref]
- Turner JA, Sears JM, Loeser JD (2007) Programmable intrathecal opioid delivery systems for chronic noncancer pain: a systematic review of effectiveness and complications. Clin J Pain 23: 180-195. [Crossref]
- Brigg GG, Freeman RK, Yaffe SJ (2002) Drug in pregnancy and lactation. Amitryptiline, alprazolam, clomipramine, diazepam, lithium, map roiling, simethicone”. Sixth edition. Philadelphia: Lippincott Williams and Wilkins Publishing 30-2.
- Bussa M, Guttilla D, Lucia M, Mascaro A, Rinaldi S (2015) Complex regional pain syndrome type I: a comprehensive review. Acta Anaesthesiol Scand 59: 685-697. [Crossref]
- Marinus J, Moseley GL, Birklein F, Baron R, Maihöfner C, et al. (2011) Clinical features and pathophysiology of complex regional pain syndrome. Lancet Neurol 10: 637-648. [Crossref]
- Sandroni P, Benrud-Larson LM, McClelland RL, Low PA (2003) Complex regional pain syndrome type I: incidence and prevalence in Olmsted county, a population-based study. Pain 103: 199-207. [Crossref]
- Shibuya N, Humphers JM, Agarwal MR, Jupiter DC (2013) Efficacy and safety of high-dose vitamin C on complex regional pain syndrome in extremity trauma and surgery--systematic review and meta-analysis. J Foot Ankle Surg 52: 62-66. [Crossref]
- de Mos M, Huygen FJ, van der Hoeven-Borgman M, Dieleman JP, Ch Stricker BH, et al. (2009) Outcome of the complex regional pain syndrome. Clin J Pain 25: 590-597. [Crossref]
- Zyluk A (2004) Complex regional pain syndrome type I. Risk factors, prevention and risk of recurrence. J Hand Surg Br 29: 334-337. [Crossref]

