Abstract
Squamous cell carcinoma (SCC) of the skin is a common cutaneous malignant tumor, with progressively increasing incidence and rising cost of management. Although most of these tumors can be effectively treated by surgical resection alone, a small fraction of patients can develop metastases and die, since there are very few effective therapeutic options for the management of metastatic SCC. Certain clinical and histopathologic parameters have been shown to correlate to some extent with aggressive behavior of SCC. However, identification of driver mutations that can be effectively targeted for therapeutic benefit has been problematic, since several studies focusing on gene expression, cytogenetic abnormalities, genomic alterations and epigenetic changes have demonstrated high mutational burden in cutaneous SCC due to chronic ultraviolet light exposure. Therefore, there is an ever-increasing need for identification of specific genetic and epigenetic markers that predict aggressive behavior and/or are targetable in cutaneous SCC. The objective of this review is to summarize the clinical and histopathologic characteristics of aggressive SCC as well as molecular events (genetic and epigenetic changes) that might contribute to squamous carcinogenesis and/or promote aggressive behavior in cutaneous SCC.
Key words
skin, squamous cell carcinoma, genes, mutations, amplifications
Introduction
Cutaneous squamous cell carcinoma (cSCC) is the 2nd most common cutaneous malignant tumor, primarily affecting the Caucasian population worldwide [1]. cSCC account for about 0.7 to 1 million cases annually in the US [2,3], of which 0.2 to 0.4 million cases are invasive, and the incidence continues to rise [4]. Recent reports show that, cSCC is the most common form of cancer among chronically immunosuppressed patients [5]. In particular, solid organ transplant recipients are at 65 to 250-fold risk of developing cSCC and their tumors tend to be more numerous, larger and exhibit greater aggressiveness compared to those in the general population [6,7]. Along with increased incidence of cSCC [8], the cost of management of these patients is also escalating rapidly [3,9].
Although cSCC constitute only 20% of all non-melanoma skin cancers or keratinocyte carcinomas, approximately 3-7% do metastasize and over 70% of patients that developed nodal metastasis died due to the disease (~2% of patients with cSCC) [4,10,11]. Prospective identification of such aggressive tumors may aid in identifying patients at high-risk for developing metastasis. Some histopathologic and clinical characteristics of the primary tumor correlate with aggressive behavior [12,13]. Identification of molecular events associated with high-risk behavior is pivotal to determining the risk of cSCC recurrence and metastasis and to determine which of these alterations could be targeted in a meaningful way. However, this process is hampered due to the presence of high background mutational burden in cSCC. We review the key clinical and histopathologic features that are typically associated with high-risk cutaneous squamous cell carcinomas (HR-cSCC) and summarize the most common and relevant molecular changes that accompany such a behavior.
Etiopathogenesis of cutaneous squamous cell carcinoma
Cutaneous SCC is a multifactorial disease, with fairly well established etiologic factors
(summarized in Table 1) that frequently play synergistic roles. Chronic exposure to the sunlight, in particular to ultraviolet-B radiation (UVR) plays a seminal role in squamous carcinogenesis [14-16]. Sun exposure has increased substantially in the past few years due to a multitude of causes including aging, recreational activities, tanning and occupational exposure [17]. There is a dose-dependent risk for developing cSCC with cumulative exposure to UVR [18,19], since UVR-induced C→T transitions and CC→TT mutations lead to generation of genotoxic bipyrimidine photoadducts [20]. In addition to direct DNA damage, UVR-induced immunomodulation is also contributory to squamous carcinogenesis [21]. Patients with Fitzpatrick types I and II skin as well as those with defective DNA repair processes are at increased risk for developing UVR-induced malignancies [22].
Table 1. Etiologic factors of cutaneous squamous cell carcinomas.
Exogenous factors |
Endogenous factors |
Radiation
Ultraviolet
Ionizing
Infections
Human Papilloma Viruses- types β and γ
Human Immunodeficiency Virus
Medications
Cytotoxic drugs
Cyclosporine
Voriconazole
Chemicals
Smoking
Arsenic
Creosote |
Immunosuppression
Primary:
Severe combined immunodeficiency
Secondary:
Chronic lymphocytic leukemia
Lymphomas
Iatrogenic: Immunosuppressive therapy for
Inflammatory disorders
Transplant: solid organ, stem cell
Genetic predisposition
Defective DNA repair mechanism:
Xeroderma pigmentosum
Rothmund Thomson syndrome
Bloom syndrome
Werner syndrome
Others:
Recessive dystrophic epidermolysis bullosa
Epidermodysplasia verruciformis
Dyskeratosis congenita
Ferguson Smith syndrome
Oculocutaneous albinism
Huriez syndrome
Chronic inflammation
Non-healing wounds
Burns
Hidradenitis suppurativa
Discoid lupus erythematosus
Osteomyelitis |
Exposure to medications such as cytotoxic or chemotherapeutic agents including azathioprine, cyclosporine and voriconazole is associated with increased risk for cSCC development [23-27], especially in chronically sun-exposed/damaged (CSD) skin. Squamous carcinogenesis has also been associated with chronic exposure to arsenic [28] and wood tar derivatives such as creosote [29]. Though a direct causative role for tobacco smoking has not been established in cSCCs, its inhibitory effect on wound healing may indirectly attribute to chronic inflammation [30] and thus, increase the risk of developing cSCC [31]. Unmitigated chronic inflammation is also an established cause of cSCC and is commonly seen in the setting of non-healing wounds secondary to burns, hidradenitis suppurativa, chronic osteomyelitis, discoid lupus erythematosus, etc. [32,33]
Primary and acquired immunodeficiency and the resulting impairment of tumor surveillance mechanisms is a major risk factor for development of cSCC [34,35]. This association is a growing concern in view of increasing number of organ transplant recipients and patients that require immunosuppressive therapy for management of inflammatory disorders such as rheumatoid arthritis [6,7]. cSCC are major health hazards in patients with hematologic malignancies such as chronic lymphocytic leukemia (CLL) and Hodgkin lymphoma [36-38]. The higher prevalence of beta and gamma type-human papilloma viral (HPV) infection in immunocompromised patients is a major predisposing factor for SCC development [39-41].
In addition, genetic predisposition for developing a range of cutaneous tumors including cSCC is well established in patients with defective DNA repair [22]. Mutations in genes encoding for structural proteins (COL7A1) [42] and transmembrane channels (EVER/TMC) [43,44] also render patients susceptible to development of intractable cSCC.
High-risk cutaneous squamous cell carcinomas
Though most tumors are amenable to cure by surgical resection, a small fraction (5-10%) of cSCCs may be difficult to excise with clear margins. They may recur locally and/or metastasize to regional lymph nodes and rarely to distant organs. Metastases are most commonly encountered within the first two years after the initial diagnosis [11,38]. Prospective identification of patients at risk for developing such high-risk cSCC could facilitate early deployment of preventive measures [45]. Several clinical and histologic features have been associated with aggressive behavior in cSCC (Table 2) and some of these have been included in the 7th edition American Joint Committee on Cancer (AJCC) staging system [12].
Table 2. Characteristics of high-risk cutaneous squamous cell carcinomas.
Clinical features |
Histopathologic features |
Age at diagnosis > 70 years
Male sex
Medications
Immunosuppressive agents
Associated conditions
Solid organ transplant
Chronic lymphocytic leukemia
Hodgkin lymphoma
Anatomic location
External ear
Preauricular area / cheek
Cutaneous lip
Temple
Nose
Dorsum of hands
Anogenital area
Non-healing wound / sites of chronic inflammation |
Tumor dimensions
Tumor diameter / size > 20.0 mm
Tumor depth > 2.0 mm
Invasion beyond subcutaneous tissue
Tumor cell type / growth pattern
Poor differentiated / grade 3
Desmoplastic features
Acantholysis
Spindled morphology
Perineural invasion
Local recurrence after surgical resection |
Clinical features
cSCC located in certain anatomic sites such as external ear, cheek, cutaneous lip, temple, nose, dorsum of hands and anogenital area tend to behave in an aggressive fashion [11,46,47]. Tumors of ear, cutaneous lip and temple are associated with increased risk for recurrence and disease-specific death; while those from ear, lip, temple and cheek tend to have a higher rate of metastasis [48]. In general, cSCC of the head and neck region have higher rates of metastasis and decreased overall and disease-specific survival [49]. One study reported that cSCC of lower extremities may require multiple resections to achieve clear margins [50]; however, high incidence of aggressive behavior have not been documented in cSCC arising at this site.
Systemic immunosuppression is associated with enhanced aggressiveness in cSCC [51]. In particular, solid organ transplant recipients are at 65 to 250-fold risk for developing greater numbers of cSCC and these tumors tend to be highly aggressive, sometimes with intractable clinical course [6,7]. Patients with CLL are at 7-17 fold increased risk for developing HR-SCC [52,53]. Local immune-deregulation in the form of persistent chronic inflammation is a major causative factor in the development of cSCC [32,33,54]. Inflammatory mediators, in particular MIF is reported to be upregulated in UVR-damaged skin as well as cSCC and inhibition of this pro-inflammatory cytokine has been reported to be beneficial at several stages of UVR-mediated squamous carcinogenesis [21].
Older age at diagnosis (over 70 years) is in general a risk-factor for having an aggressive cSCC [10]. This could be due to life-long exposure to sunlight leading to higher cumulative dose of UVR; age-associated, UVR-induced and sometimes iatrogenic reduction in immunity as well as inadequate access to or under-utilized health care. cSCC are more common in men compared to women; occupational exposure to sunlight and inherent differences in DNA damage, inflammation and repair mechanisms between male and female skin could be attributed to this dissimilarity [55]. However, it is unclear how these differences contribute to aggressive biologic behavior in males [10].
Histopathologic features
The size and thickness of the primary cSCC have been established to be important risk factors for recurrence and metastasis and have been included in the recent AJCC staging system (Figure 1) [12]. Though not always predictive, the maximum tumor diameter or size more than 20.0 mm has been associated with almost 3-fold increased risk for metastasis and 2 times propensity for local recurrence, metastasis and disease-specific survival (DSS) [48,56-58]. Also, tumors ≥ 40.0 mm were associated with increased risk for mortality [59].
While it is conceivable that greater tumor thickness might be correlated with high-risk features, multiple cut-off values have been reported in the literature to predict aggressive behavior [49,56]; for instance, >6.0 mm is correlated with increased local recurrence and shorter metastasis- free survival [57]. Recent studies have demonstrated that any tumor with depth more than 2.0 mm has increased risk for local recurrence and metastasis [48,57]. cSCC of identical tumor thickness located in different sites of the body might behave differently, depending on the thickness of reticular dermis and subcutaneous fibroadipose tissue. Therefore, invasion into reticular dermis and subcutaneous fibroadipose tissue could be potentially associated with aggressive behavior [59]. Invasion beyond subcutaneous tissue, particularly into underlying muscle or bone is associated with higher risk for metastasis [10,11,48,60].
Poor differentiation of the tumor cells is associated with 2-fold increased risk of local recurrence and 3 times risk for metastasis [46], as well as increased disease-specific mortality [48]. Predominance of acantholysis, spindled morphology and/or desmoplastic growth pattern has been shown to be more frequently associated with local recurrence and metastasis in some studies [60-63].
Perineural invasion is identified in up to 14% of all cSCC, and is particularly common and associated with worse prognosis in head and neck cSCC [64]. Perineural invasion is associated in increased risk for local recurrence (up to 47%), nodal metastasis (~35%), distant metastasis (almost 5-fold) and disease-specific death [48,64].
Local recurrence after surgical resection is frequent in cSCC in men and is often associated with the presence of perineural invasion, large dimensions and deep invasion [50]. This may necessitate several surgical resections for complete clearance with satisfactory margins. Local recurrence of cSCC is associated with increased rates of metastasis as well [56,65].
Molecular alterations in cutaneous squamous cell carcinomas
Surgical resection with clear margins is the standard of care for most cSCC cases; however, many HR-cSCC are typically not amenable surgical cure. Therefore, identification of specific molecular changes that are potentially targetable is of paramount importance for control of disease in patients with aggressive cSCC. Currently available advanced molecular screening assays have contributed to amassing inventories of molecular alterations in various neoplasms, including cSCC. However, identification of specific targetable genes, gene products and/or signaling pathways has been challenging in cSCC, due to the high mutational burden seen in these tumors secondary to chronic UVR exposure [66-70]. Therefore, differentiating oncogenic driver mutations from passenger mutations is challenging and the most frequent molecular events are reviewed below (Figure 2).
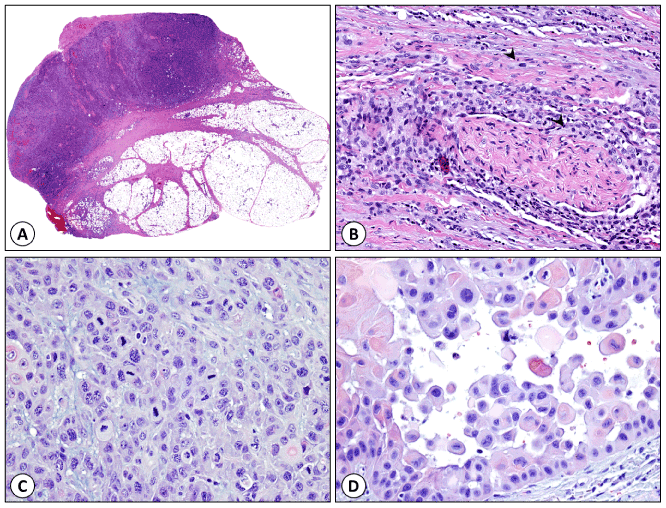
Figure 1. Histologic features of high-risk cutaneous squamous cell carcinoma. (A) Deeply invasive cSCC extending to the dermal subcutaneous interface with tumor depth of 53.0 mm. (B) Perineural and intraneural invasion (arrow heads mark tumor cells). (C) Poorly differentiated cSCC (grade 3). (D) cSCC with acantholysis.
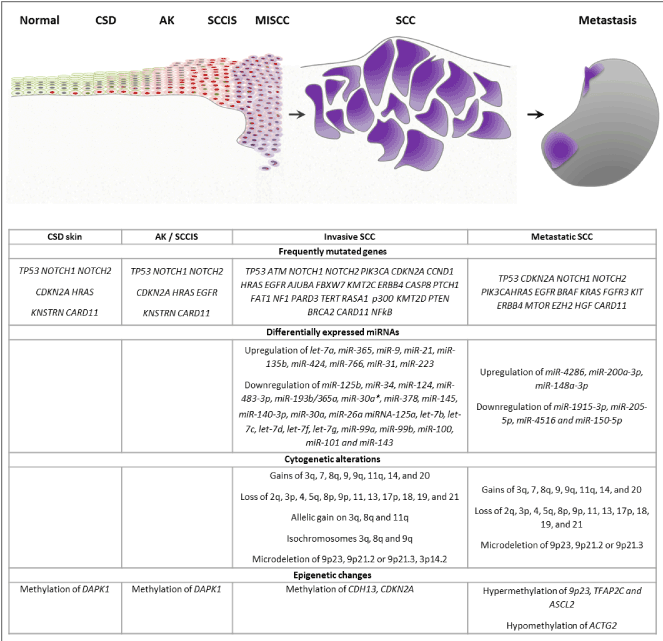
Figure 2. Schematic representing progressive acquisition of genetic and epigenetic alterations (listed below) during the development of cutaneous squamous cell carcinoma.
Frequently identified mutations in cSCC
Gene expression analyses using microarrays have revealed that several potentially oncogenic genomic alterations may exist in histologically normal CSD skin [71]. Gene expression profiles were similar between actinic keratoses (AK) and cSCC, but were found to be distinct from that of CSD and sun-shielded skin [71]. MMP1, a matrix metalloprotease, keratinocyte activation markers such as Keratin6a, transcription factors including STAT1, and the Wnt signaling cascade protein WNT5A were among genes that were upregulated in cSCC compared to normal skin. In contrast, IGFL1, markers of differentiating spinous keratinocytes such as SPRR1A, SPRR1B and involucrin were differentially expressed in AKs when compared to CSD skin and cSCC [72]. Bioinformatics analyses highlight several signaling pathways operative in SCC and enrichment of adhesion proteins such as integrins α2 and α6, MMP1, inflammatory mediators such as IL8, and HIF1 to be upregulated in SCC [73].
Next generation sequencing (NGS) analyses have revealed that though cSCC are predominantly driven by UVR exposure, they share similar molecular alterations with SCC arising in other parts of the body [74], compared to other non-squamous cell carcinomas. The most frequently mutated genes included PIK3CA, CCND1, CDKN2A, SOX2, NOTCH1, and FBXW7, while KRAS mutations were conspicuously absent in SCCs, suggesting that this gene alteration profile imparted ‘squamousness’ to these tumors. The authors also identified 2 molecularly divergent groups of SCCs containing either p53 and cyclin pathway alterations or PIK3CA mutations.
Whole exome sequencing (WES) on fresh-frozen tumor tissue from aggressive cSCC arising from head and neck skin (metastatic and/or displaying high-risk features) revealed mutations involving 23 genes including TP53, CDKN2A, NOTCH1, NOTCH2, AJUBA, HRAS, CASP8, FAT1, KMT2C, PARD3, RASA1 and RIPK4 [70]. Of these KMT2C mutations were associated with increased risk for bone invasion and hence, a worse prognosis. Sequencing of advanced cSCC (77 primary and 45 metastatic tumors) identified TP53, CDKN2A, NOTCH1, KMT2D, LRPIB, and TERT to be commonly mutated (in approximately 20% of the tumors tested) [68]. Other clinically relevant mutations were identified involving PTCH1, BRCA2, HRAS, ATM, ERBB4, NF1, PIK3CA, CCND1, EGFR and FBXW7 genes. Sequencing of 29 metastatic cSCC also confirmed the presence of commonly identified mutations involving TP53, CDKN2A, NOTCH1 and NOTCH2 [69]. Functional mutations of BRAF, KRAS, FGFR3, KIT, HRAS, EGFR, ERBB4, EZH2, MTOR, PIK3CA, HGF, and CARD11 were also detected in some cases and several of these are actionable.
Commonly mutated genes in cutaneous squamous cell carcinomas
Data from the genomic analyses mentioned above have identified some genes to be frequently mutated in cSCC. For instance, more than 90% of cSCCs and their precursors including most AKs harbor UVR induced TP53 mutations [75-77], which are easily detectable by immunostains, even in histologically unremarkable CSD skin [78]. Loss-of-function mutation involving both p53 alleles is a critical molecular event that triggers transformation of AK into cSCC [79]. The level of p53 protein expression in SCCs have also been correlated to the histologic grade, high-risk features, and therefore, to the stage and prognosis [80].
NOTCH is a direct target of p53 contributing to differentiation of epidermal keratinocytes; while NOTCH1 is expressed throughout the epidermis, NOTCH2 is localized principally to the basal layer [81-83]. Inactivation of NOTCH1 or NOTCH2 through point mutations in functional domains as well as truncation mutations is a common event in cSCC and has been identified in more than 75% of these tumors [84]. NOTCH1 mutation is an early event in squamous carcinogenesis of the skin and has been identified as patchy loss of expression in histologically normal epidermis [66], and can be induced by exposure to UVR [85]. Inhibition of NOTCH by HPV-derived proteins also contributes to oncogenic transformation of keratinocytes [86].
CDKN2A encodes for p16INK4a and p14ARF, of which inactivation of p16INK4a (also referred to as p16) by loss of heterozygosity (LOH), mutations and homozygous deletions has been associated with progression of cSCC from AK and increased expression of p16 has been reported in up to 50% of all cSCC [87-89]. Gain-of-function mutations of HRAS have been identified in up to 20% of cSCC [66,68,79]. However, the incidence of HRAS mutations is more prevalent in squamous lesions developing in patients receiving BRAF inhibitor therapy (~40%) [90]. EGFR overexpression is a common feature of SCC and EGFR activation appears to be an early event in squamous carcinogenesis, more than 50% of AKs and ~70% of cSCC exhibit several EGFR signals, while EGFR amplification was identified 12% of AK [91]. EGFR inhibitor therapy has shown promise in treatment of aggressive cSCC in the neoadjuvant setting [92].
Increased expression of the transcriptional coactivator p300 is common in aggressive cSCC and has been associated with shorter recurrence-free-survival and overall survival in these patients [93]. UVR-induced mutations are common in cSCC compared to SCC of other organs and are identified in 50-70% of cSCC cases [94,95]. UVR-signature mutations in KNSTRN, a kinetochore protein have also been detected in up to 19% of cSCC [96]. Point mutations of KNSTRN affect chromatid adhesion, leading to aneuploidy in keratinocytes, a feature associated with high-risk cSCC [11]. These mutations can be identified in histologically normal epidermis, AK and cSCC. CARD11 is a scaffold protein that regulates NFkB signaling cascade; point mutations of CARD11 can lead to constitutive activation of the NFkB pathway, which in turn contributes to transformation of keratinocytes [97]. CARD11 mutations are identified in the surrounding histologically normal epidermis, suggesting that these are also early events in squamous carcinogenesis.
MAPK and PI3K/AKT pathways are mostly commonly altered in cSCC. Altered expression of TP63, NOTCH-1,2,4 RIPK4, CARD11, in conjunction with CREBBP and p300 enhance proliferation while blocking terminal differentiation of keratinocytes, leading to malignant transformation. Inactivating mutations of p53 and rarely Rb as well as activating mutations of cyclinD1 provide survival advantage and resistance to apoptosis to the transformed keratinocytes [69].
Chromosomal alterations in cSCC
Single nucleotide polymorphism (SNP) assays reveal fewer genomic alterations in well-differentiated SCC compared to moderately and poorly differentiated tumors [78]; allelic imbalances were identified in a median of 5 and 9 chromosomes in well-differentiated and moderately or poorly differentiated tumors, respectively. Copy number gains were identified on chromosomes 3q, 5q, 7, 8q, 9q, 11q (CCND1), 14, and 20, while loss of 3p, 4, 5q, 8p, 9p, 11, 17p, 18, 19, and 21 were frequent [70]. Genome-wide association study of primary cSCC identified SNPs in 10 common loci, 6 of which contained genes controlling pigmentation such as SLC45A2, IRF4, TYR, HERC2/OCA2, DEF8 and RALY [98]. FOXP1, TP63, HLA-DQA1 and BNC2 were located at regions of other SNPs.
Epigenetic changes in cSCC
Loss-of-heterozygosity (LOH) at 9p was the most frequent epigenetic event identified in approximately 75% of cSCCs, while the 2nd most common event was LOH at 3p was found in 65% of the cases [78]. Homozygous microdeletions at 9p23, corresponding to PTPRD locus was identified in 15% of the examined cSCC and most of these tumors were poorly differentiated, while only a few of those had given rise to metastasis. In addition, homozygous microdeletions at 9p21.2 or 9p21.3 were also identified, corresponding to the CDKN2A locus. Microdeletions at 3p14.2, were also detected in some cSCCs in conjunction with LOH of the other allele and this was the location of FHIT gene. Other frequent loci of LOH included 13q, 8p and 9q. Analysis of methylation status of specific genes revealed that methylation of DAPK1 promoter was identified in the tumor as well as the surrounding tissues, suggesting that this was an early event in squamous carcinogenesis [99]. On the other hand, methylation of CDKN2A and CDH13 gene promoters were detected most frequently in the tumor tissue. Analysis of epigenetic profiles of metastatic cSCC identified methylation at FRZB, a member of the Wnt signaling pathway to be the most common event [100], in addition to transcription factors TFAP2C and ASCL2, while ACTG2 was hypomethylated.
MicroRNAs in cSCC
MicroRNAs are non-coding RNAs that regulate post-transcriptional gene expression and miRNA profiling studies have revealed altered expression of miRNAs in cSCC. For instance, Let-7a targeting caspase3 is upregulated in SCC, while miR-9 is over-expressed in SCC and metastases and is associated with loss of expression of α-catenin [101]. miR-21 is also upregulated in cSCC and psoriasis, resulting in decreased expression of MSH2, DND1, GRHL3 and PTEN, leading to aggressive behavior [102]. miR-365 is another microRNA that is upregulated in SCC and other carcinomas that functions by mediating the downregulation of nuclear factor, NF-I/B [103]. miRNAs that are specifically over-expressed in cSCC include miR-135b, miR-424 and miR-766 [104], miR-31 and miR-223 [105]. MicroRNAs that are specifically downregulated in cSCC include miR-125b, miR-34a, miR-124, miR-483-3p, and miR-193b/365a targeting MMP13, SIRT6, ERK1/2, CDC25A and KRAS, respectively [102]. miR-30a*, miR-378, miR-145, miR-140-3p, miR-30a, miR-26a, miR-375, miRNA-125a, let-7b, let-7c, let-7d, let-7f, let-7g, miR-99a, miR-99b, miR-100, miR-101 and miR-143 levels are significantly reduced in cSCC compared to histologically normal tissue [104,105]. Comparison of primary and metastatic cSCC revealed that miR-4286, miR-200a-3p, miR-148a-3p were upregulated while, miR-1915-3p, miR-205-5p, miR-4516 and miR-150-5p were down-regulated specifically in the metastatic tumors [106].
Summary
Carcinogenic transformation of epidermal keratinocytes into cutaneous squamous cell carcinoma is a complex process involving the interaction of numerous gene products converging in signaling cascades that regulate several biologic processes. Deregulation of several checkpoints is necessary for carcinogenic transformation. Ultraviolet radiation, particularly its B-fraction functions as complete carcinogen by promoting DNA-damage, sustaining such altered cells, promoting their proliferation while preventing apoptosis, lowering immune response and thus, promoting carcinogenic transformation. Altered expression and function of several other proteins is critical before the actual tumorigenesis begins. While our understanding of this complex interplay has increased by leaps and bounds in the past few decades, identification of clinically relevant and targetable genetic alterations has not been very successful so far, but is essential for optimal treatment of these patients.
References
- LeBoeuf NR, Schmults CD (2011) Update on the management of high-risk squamous cell carcinoma. Semin Cutan Med Surg 30: 26-34. [Crossref]
- Rogers HW, Weinstock MA, Harris AR, Hinckley MR, Feldman SR, et al. (2010) Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol 146: 283-287. [Crossref]
- Guy GP Jr, Machlin SR, Ekwueme DU, Yabroff KR4 (2015) Prevalence and costs of skin cancer treatment in the U.S., 2002-2006 and 2007-2011. Am J Prev Med 48: 183-187. [Crossref]
- Karia PS, Han J, Schmults CD (2013) Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol 68: 957-966. [Crossref]
- Uribe P, Gonzalez S (2011) Epidermal growth factor receptor (EGFR) and squamous cell carcinoma of the skin: molecular bases for EGFR-targeted therapy. Pathol Res Pract 207: 337-342. [Crossref]
- Rangwala S, Tsai KY (2011) Roles of the immune system in skin cancer. Br J Dermatol 165: 953-965. [Crossref]
- Colegio OR, Billingsley EM (2011) Skin cancer in transplant recipients, out of the woods. Scientific retreat of the ITSCC and SCOPE. Am J Transplant 11: 1584-1591. [Crossref]
- Lomas A, Leonardi-Bee J, Bath-Hextall F (2012) A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol 166: 1069-1080. [Crossref]
- Housman TS, Feldman SR, Williford PM, Fleischer AB Jr, Goldman ND, et al. (2003) Skin cancer is among the most costly of all cancers to treat for the Medicare population. J Am Acad Dermatol 48: 425-429. [Crossref]
- Schmults CD, Karia PS, Carter JB, Han J, Qureshi AA (2013) Factors predictive of recurrence and death from cutaneous squamous cell carcinoma: a 10-year, single-institution cohort study. JAMA Dermatol 149: 541-547. [Crossref]
- Weinberg AS, Ogle CA, Shim EK (2007) Metastatic cutaneous squamous cell carcinoma: an update. Dermatol Surg 33: 885-899. [Crossref]
- Farasat S, Yu SS, Neel VA, Nehal KS, Lardaro T, et al. (2011) A new American Joint Committee on Cancer staging system for cutaneous squamous cell carcinoma: creation and rationale for inclusion of tumor (T) characteristics. J Am Acad Dermatol 64: 1051-1059. [Crossref]
- Goldenberg A, Ortiz A, Kim SS, Jiang SB (2015) Squamous cell carcinoma with aggressive subclinical extension: 5-year retrospective review of diagnostic predictors. J Am Acad Dermatol 73: 120-126. [Crossref]
- Narayanan DL, Saladi RN, Fox JL (2010) Ultraviolet radiation and skin cancer. Int J Dermatol 49: 978-986. [Crossref]
- Centers for Disease Control and Prevention (CDC) (2012) Sunburn and sun protective behaviors among adults aged 18-29 years--United States, 2000-2010. MMWR Morb Mortal Wkly Rep 61: 317-322. [Crossref]
- Rigel DS (2008) Cutaneous ultraviolet exposure and its relationship to the development of skin cancer. J Am Acad Dermatol 58: S129-132. [Crossref]
- Donaldson MR, Coldiron BM (2011) No end in sight: the skin cancer epidemic continues. Semin Cutan Med Surg 30: 3-5. [Crossref]
- Gallagher RP, Lee TK, Bajdik CD, Borugian M (2010) Ultraviolet radiation. Chronic Dis Can 29 Suppl 1: 51-68. [Crossref]
- El Ghissassi F, Baan R, Straif K, Grosse Y, Secretan B, et al. (2009) A review of human carcinogens--part D: radiation. Lancet Oncol 10: 751-752. [Crossref]
- Cadet J, Mouret S, Ravanat JL, Douki T (2012) Photoinduced damage to cellular DNA: direct and photosensitized reactions. Photochem Photobiol 88: 1048-1065. [Crossref]
- Nagarajan P, Tober KL, Riggenbach JA, Kusewitt DF, Lehman AM, et al. (2014) MIF antagonist (CPSI-1306) protects against UVB-induced squamous cell carcinoma. Mol Cancer Res 12: 1292-1302. [Crossref]
- Jaju PD, Ransohoff KJ, Tang JY, Sarin KY (2016) Familial skin cancer syndromes: Increased risk of nonmelanotic skin cancers and extracutaneous tumors. J Am Acad Dermatol 74: 437-451. [Crossref]
- Epaulard O, Villier C, Ravaud P, Chosidow O, Blanche S, et al. (2013) A multistep voriconazole-related phototoxic pathway may lead to skin carcinoma: results from a French nationwide study. Clin Infect Dis 57: e182-188. [Crossref]
- Walsh SB, Xu J, Xu H, Kurundkar AR, Maheshwari A, et al. (2011) Cyclosporine a mediates pathogenesis of aggressive cutaneous squamous cell carcinoma by augmenting epithelial-mesenchymal transition: role of TGFbeta signaling pathway. Mol Carcinog 50: 516-527. [Crossref]
- Williams K, Mansh M, Chin-Hong P, Singer J, Arron ST (2014) Voriconazole-associated cutaneous malignancy: a literature review on photocarcinogenesis in organ transplant recipients. Clin Infect Dis 58: 997-1002. [Crossref]
- Coghill AE, Johnson LG, Berg D, Resler AJ2,, et al. (2016) Immunosuppressive Medications and Squamous Cell Skin Carcinoma: Nested Case-Control Study Within the Skin Cancer after Organ Transplant (SCOT) Cohort. Am J Transplant 16: 565-573. [Crossref]
- Ingvar A, Smedby KE, Lindelöf B, Fernberg P, Bellocco R, et al. (2010) Immunosuppressive treatment after solid organ transplantation and risk of post-transplant cutaneous squamous cell carcinoma. Nephrol Dial Transplant 25: 2764-2771. [Crossref]
- Cabrera HN, Gómez ML (2003) Skin cancer induced by arsenic in the water. J Cutan Med Surg 7: 106-111. [Crossref]
- Karlehagen S, Andersen A, Ohlson CG (1992) Cancer incidence among creosote-exposed workers. Scand J Work Environ Health 18: 26-29. [Crossref]
- Thomsen SF, Sørensen LT (2010) Smoking and skin disease. Skin Therapy Lett 15: 4-7. [Crossref]
- De Hertog SA, Wensveen CA, Bastiaens MT, Kielich CJ, Berkhout MJ, et al. (2001) Relation between smoking and skin cancer. J Clin Oncol 19: 231-238. [Crossref]
- Lavogiez C, Delaporte E, Darras-Vercambre S, Martin De Lassalle E, Castillo C, et al. (2010) Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology 220: 147-153. [Crossref]
- Katz RD, Goldberg NH (2009) Marjolin ulcer arising within hidradenitis: a case report and literature review. Ann Plast Surg 62: 173-174. [Crossref]
- Yu SH, Bordeaux JS, Baron ED (2014) The immune system and skin cancer. Adv Exp Med Biol 810: 182-191. [Crossref]
- Strickland FM, Kripke ML (1997) Immune response associated with nonmelanoma skin cancer. Clin Plast Surg 24: 637-647. [Crossref]
- Tsimberidou AM, Wen S, McLaughlin P, O'Brien S, Wierda WG, et al. (2009) Other malignancies in chronic lymphocytic leukemia/small lymphocytic lymphoma. J Clin Oncol 27: 904-910. [Crossref]
- Brewer JD, Shanafelt TD, Khezri F, Sosa Seda IM, Zubair AS, et al. (2015) Increased incidence and recurrence rates of nonmelanoma skin cancer in patients with non-Hodgkin lymphoma: a Rochester Epidemiology Project population-based study in Minnesota. J Am Acad Dermatol 72: 302-309. [Crossref]
- Tchernev G, Ananiev J, Semkova K, Dourmishev LA, Schönlebe J, et al. (2013) Nevus comedonicus: an updated review. Dermatol Ther (Heidelb) 3: 33-40. [Crossref]
- Quint KD, Genders RE, de Koning MN, Borgogna C, Gariglio M, et al. (2015) Human Beta-papillomavirus infection and keratinocyte carcinomas. J Pathol 235: 342-354. [Crossref]
- Wang J, Aldabagh B, Yu J, Arron ST4 (2014) Role of human papillomavirus in cutaneous squamous cell carcinoma: a meta-analysis. J Am Acad Dermatol 70: 621-629. [Crossref]
- Reusser NM, Downing C, Guidry J, Tyring SK4 (2015) HPV Carcinomas in Immunocompromised Patients. J Clin Med 4: 260-281. [Crossref]
- Kim M, Murrell DF (2021 Copyright OAT. All rights reservquamous cell carcinoma development in recessive dystrophic epidermolysis bullosa. Eur J Dermatol 25 Suppl 1: 30-32. [Crossref]
- Burger B, Itin PH (2014) Epidermodysplasia verruciformis. Curr Probl Dermatol 45: 123-131. [Crossref]
- Hampras SS, Rollison DE, Tommasino M, Gheit T, Schabath MB, et al. (2015) Genetic variations in the epidermodysplasia verruciformis (EVER/TMC) genes, cutaneous human papillomavirus infection and squamous cell carcinoma of the skin. Br J Dermatol 173: 1532-1535. [Crossref]
- Mavropoulos JC, Aldabagh B, Arron ST (2014) Prospects for personalized targeted therapies for cutaneous squamous cell carcinoma. Semin Cutan Med Surg 33: 72-75. [Crossref]
- Dinehart SM, Pollack SV (1989) Metastases from squamous cell carcinoma of the skin and lip. An analysis of twenty-seven cases. J Am Acad Dermatol 21: 241-248. [Crossref]
- Kraus DH, Carew JF, Harrison LB (1998) Regional lymph node metastasis from cutaneous squamous cell carcinoma. Arch Otolaryngol Head Neck Surg 124: 582-587. [Crossref]
- Thompson AK, Kelley BF, Prokop LJ, Murad MH, Baum CL (2016) Risk Factors for Cutaneous Squamous Cell Carcinoma Recurrence, Metastasis, and Disease-Specific Death: A Systematic Review and Meta-analysis. JAMA Dermatol 152: 419-428. [Crossref]
- Moore BA, Weber RS, Prieto V, El-Naggar A, Holsinger FC, et al. (2005) Lymph node metastases from cutaneous squamous cell carcinoma of the head and neck. Laryngoscope 115: 1561-1567. [Crossref]
- Dzubow LM, Rigel DS, Robins P (1982) Risk factors for local recurrence of primary cutaneous squamous cell carcinomas. Treatment by microscopically controlled excision. Arch Dermatol 118: 900-902. [Crossref]
- Marrazzo G, Thorpe R, Condie D, Pinho MC, Srivastava D (2015) Clinical and Pathologic Factors Predictive of Positive Radiologic Findings in High-Risk Cutaneous Squamous Cell Carcinoma. Dermatol Surg 41: 1405-1410. [Crossref]
- Mehrany K, Weenig RH, Pittelkow MR, Roenigk RK, Otley CC (2005) High recurrence rates of squamous cell carcinoma after Mohs' surgery in patients with chronic lymphocytic leukemia. Dermatol Surg 31: 38-42. [Crossref]
- Royle JA, Baade PD, Joske D, Girschik J, Fritschi L (2011) Second cancer incidence and cancer mortality among chronic lymphocytic leukaemia patients: a population-based study. Br J Cancer 105: 1076-1081. [Crossref]
- Motswaledi MH, Khammissa RA, Wood NH, Meyerov R, Lemmer J, et al. (2011) Discoid lupus erythematosus as it relates to cutaneous squamous cell carcinoma and to photosensitivity. SADJ 66: 340-343. [Crossref]
- Oberyszyn TM (2008) Non-melanoma skin cancer: importance of gender, immunosuppressive status and vitamin D. Cancer Lett 261: 127-136. [Crossref]
- Rowe DE, Carroll RJ, Day CL Jr (1992) Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J Am Acad Dermatol 26: 976-990. [Crossref]
- Brantsch KD, Meisner C, Schönfisch B, Trilling B, Wehner-Caroli J, et al. (2008) Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol 9: 713-720. [Crossref]
- Nuño-González A, Vicente-Martín FJ, Pinedo-Moraleda F, López-Estebaranz JL (2012) High-risk cutaneous squamous cell carcinoma. Actas Dermosifiliogr 103: 567-578. [Crossref]
- Clayman GL, Lee JJ, Holsinger FC, Zhou X, Duvic M, et al. (2005) Mortality risk from squamous cell skin cancer. J Clin Oncol 23: 759-765. [Crossref]
- Breuninger H, Black B, Rassner G (1990) Microstaging of squamous cell carcinomas. Am J Clin Pathol 94: 624-627. [Crossref]
- Evans HL, Smith JL (1980) Spindle cell squamous carcinomas and sarcoma-like tumors of the skin: a comparative study of 38 cases. Cancer 45: 2687-2697. [Crossref]
- Nappi O, Pettinato G, Wick MR (1989) Adenoid (acantholytic) squamous cell carcinoma of the skin. J Cutan Pathol 16: 114-121. [Crossref]
- Garcia C, Crowson AN (2011) Acantholytic squamous cell carcinoma: is it really a more-aggressive tumor? Dermatol Surg 37: 353-356. [Crossref]
- Goepfert H, Dichtel WJ, Medina JE, Lindberg RD, Luna MD (1984) Perineural invasion in squamous cell skin carcinoma of the head and neck. Am J Surg 148: 542-547. [Crossref]
- Tavin E, Persky M (1996) Metastatic cutaneous squamous cell carcinoma of the head and neck region. Laryngoscope 106: 156-158. [Crossref]
- South AP, Purdie KJ, Watt SA, Haldenby S, den Breems NY, et al. (2014) NOTCH1 mutations occur early during cutaneous squamous cell carcinogenesis. J Invest Dermatol 134: 2630-2638. [Crossref]
- Harwood CA, Proby CM, Inman GJ, Leigh IM (2016) The Promise of Genomics and the Development of Targeted Therapies for Cutaneous Squamous Cell Carcinoma. Acta Derm Venereol 96: 3-16. [Crossref]
- Al-Rohil RN, Tarasen AJ, Carlson JA, Wang K, Johnson A, et al. (2016) Evaluation of 122 advanced-stage cutaneous squamous cell carcinomas by comprehensive genomic profiling opens the door for new routes to targeted therapies. Cancer 122: 249-257. [Crossref]
- Li YY, Hanna GJ, Laga AC, Haddad RI, Lorch JH, et al. (2015) Genomic analysis of metastatic cutaneous squamous cell carcinoma. Clin Cancer Res 21: 1447-1456. [Crossref]
- Pickering CR, Zhou JH, Lee JJ, Drummond JA, Peng SA, et al. (2014) Mutational landscape of aggressive cutaneous squamous cell carcinoma. Clin Cancer Res 20: 6582-6592. [Crossref]
- Padilla RS, Sebastian S, Jiang Z, Nindl I, Larson R (2010) Gene expression patterns of normal human skin, actinic keratosis, and squamous cell carcinoma: a spectrum of disease progression. Arch Dermatol 146: 288-293. [Crossref]
- Ra SH, Li X, Binder S (2011) Molecular discrimination of cutaneous squamous cell carcinoma from actinic keratosis and normal skin. Mod Pathol 24: 963-973. [Crossref]
- Shen LI, Liu L, Yang Z, Jiang N (2016) Identification of genes and signaling pathways associated with squamous cell carcinoma by bioinformatics analysis. Oncol Lett 11: 1382-1390. [Crossref]
- Schwaederle M, Elkin SK, Tomson BN, Carter JL, Kurzrock R (2015) Squamousness: Next-generation sequencing reveals shared molecular features across squamous tumor types. Cell Cycle 14: 2355-2361. [Crossref]
- Kress S, Sutter C, Strickland PT, Mukhtar H, Schweizer J, et al. (1992) Carcinogen-specific mutational pattern in the p53 gene in ultraviolet B radiation-induced squamous cell carcinomas of mouse skin. Cancer Res 52: 6400-6403. [Crossref]
- Hudson AR, Antley CM, Kohler S, Smoller BR (1999) Increased p53 staining in normal skin of posttransplant, immunocompromised patients and implications for carcinogenesis. Am J Dermatopathol 21: 442-445. [Crossref]
- Bolshakov S, Walker CM, Strom SS, Selvan MS, Clayman GL, et al. p53 mutations in human aggressive and nonaggressive basal and squamous cell carcinomas. Clin Cancer Res 9: 228-234. [Crossref]
- Purdie KJ, Harwood CA, Gulati A, Chaplin T, Lambert SR, et al. (2009) Single nucleotide polymorphism array analysis defines a specific genetic fingerprint for well-differentiated cutaneous SCCs. J Invest Dermatol 129: 1562-1568. [Crossref]
- Durinck S, Ho C, Wang NJ, Liao W, Jakkula LR, et al. (2011) Temporal dissection of tumorigenesis in primary cancers. Cancer Discov 1: 137-143. [Crossref]
- Bukhari MH, Niazi S, Chaudhry NA (2009) Relationship of immunohistochemistry scores of altered p53 protein expression in relation to patient's habits and histological grades and stages of squamous cell carcinoma. J Cutan Pathol 36: 342-349. [Crossref]
- Okuyama R, Tagami H, Aiba S (2008) Notch signaling: its role in epidermal homeostasis and in the pathogenesis of skin diseases. J Dermatol Sci 49: 187-194. [Crossref]
- Chin SS, Romano RA, Nagarajan P, Sinha S, Garrett-Sinha LA (2013) Aberrant epidermal differentiation and disrupted ΔNp63/Notch regulatory axis in Ets1 transgenic mice. Biol Open 2: 1336-1345. [Crossref]
- Ota T, Takekoshi S, Takagi T, Kitatani K, Toriumi K, et al. (2014) Notch signaling may be involved in the abnormal differentiation of epidermal keratinocytes in psoriasis. Acta Histochem Cytochem 47: 175-183. [Crossref]
- Wang NJ, Sanborn Z, Arnett KL, Bayston LJ, Liao W, et al. (2011) Loss-of-function mutations in Notch receptors in cutaneous and lung squamous cell carcinoma. Proceedings of the National Academy of Sciences of the United States of America 108: 17761-17766.
- Panelos J, Tarantini F, Paglierani M, Di Serio C, Maio V, et al. (2008) Photoexposition discriminates Notch 1 expression in human cutaneous squamous cell carcinoma. Mod Pathol 21: 316-325. [Crossref]
- Connolly K, Manders P, Earls P, Epstein RJ (2014) Papillomavirus-associated squamous skin cancers following transplant immunosuppression: one Notch closer to control. Cancer Treat Rev 40: 205-214. [Crossref]
- Eshkoor SA, Ismail P, Rahman SA, Mirinargesi M, Oshkour SA (2009) Increased protein expression of p16 and cyclin D1 in squamous cell carcinoma tissues. Biosci Trends 3: 105-109. [Crossref]
- Gray SE, Kay E, Leader M, Mabruk M (2006) Analysis of p16 expression and allelic imbalance / loss of heterozygosity of 9p21 in cutaneous squamous cell carcinomas. J Cell Mol Med 10: 778-788. [Crossref]
- Brown VL, Harwood CA, Crook T, Cronin JG, Kelsell DP, et al. (2004) p16INK4a and p14ARF tumor suppressor genes are commonly inactivated in cutaneous squamous cell carcinoma. J Invest Dermatol 122: 1284-1292. [Crossref]
- Lacouture ME, Duvic M, Hauschild A, Prieto VG, Robert C, et al. (2013) Analysis of dermatologic events in vemurafenib-treated patients with melanoma. Oncologist 18: 314-322. [Crossref]
- Toll A, Salgado R, Yébenes M, Martín-Ezquerra G, Gilaberte M, et al. (2010) Epidermal growth factor receptor gene numerical aberrations are frequent events in actinic keratoses and invasive cutaneous squamous cell carcinomas. Exp Dermatol 19: 151-153. [Crossref]
- Lewis CM, Glisson BS, Feng L, Wan F, Tang X, et al. (2012) A phase II study of gefitinib for aggressive cutaneous squamous cell carcinoma of the head and neck. Clin Cancer Res 18: 1435-1446. [Crossref]
- Chen MK, Cai MY, Luo RZ, Tian X, Liao QM, et al. (2015) Overexpression of p300 correlates with poor prognosis in patients with cutaneous squamous cell carcinoma. Br J Dermatol 172: 111-119. [Crossref]
- Cheng KA, Kurtis B, Babayeva S, Zhuge J, Tantchou I, et al. (2015) Heterogeneity of TERT promoter mutations status in squamous cell carcinomas of different anatomical sites. Ann Diagn Pathol 19: 146-148. [Crossref]
- Griewank KG, Murali R, Schilling B, Schimming T, Möller I, t al. (2013) TERT promoter mutations are frequent in cutaneous basal cell carcinoma and squamous cell carcinoma. PloS one 8: e80354. [Crossref]
- Lee CS, Bhaduri A, Mah A, Johnson WL, Ungewickell A, et al. (2014) Recurrent point mutations in the kinetochore gene KNSTRN in cutaneous squamous cell carcinoma. Nat Genet 46: 1060-1062. [Crossref]
- Watt SA, Purdie KJ, den Breems NY, Dimon M, Arron ST, et al. (2015) Novel CARD11 Mutations in Human Cutaneous Squamous Cell Carcinoma Lead to Aberrant NF-κB Regulation. Am J Pathol 185: 2354-2363. [Crossref]
- Asgari MM, Wang W, Ioannidis NM, Itnyre J, Hoffmann T, et al. (2016) Identification of Susceptibility Loci for Cutaneous Squamous Cell Carcinoma. J Invest Dermatol 136: 930-937. [Crossref]
- Li L, Jiang M, Feng Q, Kiviat NB, Stern JE, et al. (2015) Aberrant Methylation Changes Detected in Cutaneous Squamous Cell Carcinoma of Immunocompetent Individuals. Cell Biochem Biophys . [Crossref]
- Darr OA, Colacino JA, Tang AL, McHugh JB, Bellile EL, et al. (2015) Epigenetic alterations in metastatic cutaneous carcinoma. Head Neck 37: 994-1001. [Crossref]
- Tsang WP, Kwok TT (2008) Let-7a microRNA suppresses therapeutics-induced cancer cell death by targeting caspase-3. Apoptosis 13: 1215-1222. [Crossref]
- Syed DN, Lall RK, Mukhtar H (2015) MicroRNAs and photocarcinogenesis. Photochem Photobiol 91: 173-187. [Crossref]
- Zhou M, Zhou L, Zheng L, Guo L, Wang Y, et al. (2014) miR-365 promotes cutaneous squamous cell carcinoma (CSCC) through targeting nuclear factor I/B (NFIB). PLoS One 9: e100620. [Crossref]
- Sand M, Skrygan M, Georgas D, Sand D, Hahn SA, et al. (2012) Microarray analysis of microRNA expression in cutaneous squamous cell carcinoma. J Dermatol Sci 68: 119-126. [Crossref]
- Bruegger C, Kempf W, Spoerri I, Arnold AW, Itin PH, et al. (2013) MicroRNA expression differs in cutaneous squamous cell carcinomas and healthy skin of immunocompetent individuals. Exp Dermatol 22: 426-428. [Crossref]
- Gillespie J, Skeeles LE, Allain DC, Kent MN, Peters SB, et al. (2015) MicroRNA expression profiling in metastatic cutaneous squamous cell carcinoma. J Eur Acad Dermatol Venereol. [Crossref]


