Atrial fibrillation (AF) is well-defined as a cardiac arrhythmia with the following features: superficial ECG displays ‘absolutely’ unequal RR intervals (AF is consequently at times recognized as arrhythmia absoluta), i.e., RR intervals that do not follow a repetitive form; there are no different P waves on the superficial ECG. Some apparently systematic atrial electrical activity may be perceived in some ECG leads, most frequently in lead V1; the atrial cycle measurement (when visible), i.e., the interval between two atrial activations, is typically variable and, <200 ms (>300 bpm). An irregular pulse should always raise the suspicion of AF, but an ECG recording is necessary to diagnose AF. Any arrhythmia that has the ECG features of AF and persists sufficiently extended for a 12-lead ECG to be documented, or at least 30 s on a rhythm stripe, should be considered as AF [1,2]. Silent AF (asymptomatic) may manifest as an AF-related complication (ischemic stroke or tachycardiomyopathy) or may be established by an opportunistic ECG. Silent AF may present as any of the temporal forms of AF [3]. Our study examined patients who had a DDDR pacemaker aiming to determine the number of patients that presents silent episodes of AF and never had an AF event detected before, in subjects that already suffered a stroke or/and transient ischemic attack (TIA) previously.
The present observational, prospective study was conducted at the Department of Cardiac Pacing and Cardiac Surgery of the Hospital e Clínica São Gonçalo. A cohort of patients received standard therapy for treatment of distal atrioventricular (AV) block and DDDR pacemaker implantation. Follow-up was done during 18 months after the implantation procedure. The study inclusion criteria were as follows: (i) patients did not have electrocardiogram-documented AF or a previous history of paroxysmal AF; (ii) patients that provided documentation of no cardiac ischemia before pacemaker implantation as proven by a myocardial scintigraphy at rest and during stress, a cardiac magnetic resonance imaging at rest and during stress, or pharmacological stress echocardiography; (iii) tests showing that patients had third- or second-degree Mobitz II AV block; (iv) patients between 18 and 95 years; (v) subjects that already suffered stroke or/and transient ischemic attack (TIA) previously; (vi) and the ability to read, understand and sign the informed consent form, besies attend the study. Exclusion criteria were as follows: (i) ischemic heart disease; (ii) the left atrial volume higher than 69 mL in men and 54 mL in women; (iii) heart valvar disease that may lead to AF; and (iv) symptoms suggestive of AF.
AF was defined as at least one episode of atrial irregular activity recorded by the atrial channel lasting ≥30 s [3]. Enrolment of patients started in January 2009 and was terminated in September 2014. Patients were followed up until July 2016, and they were identified at our offices. The study was performed in accordance with the Helsinki Declaration and was approved by the Ethics Committee of our hospital. Each patient provided written informed consent before to be included. As a routine practice in our department, bipolar leads were implanted in the appendage of the right atrium and in the high septal region of the right ventricle. DDDR pacemakers from St. Jude Medical (St. Jude Medical, St. Paul, Minnesota, USA) and Medtronic (Medtronic, Palo Alto, CA, USA) were used. The rate adaptive function was activated in all the pacemakers and programed with an inferior rate of 60 bpm and a higher rate of 120 bpm in all the pacemakers, we programed the paced atrioventricular interval to 140–220 ms and turned on the AV delay management algorithm that automatically searches for intrinsic conduction to prevent unnecessary right ventricular pacing. The maximum tracking rate was individualized, and the auto mode switch (AMS) function was activated. AMS occurred when the atrial rate exceeded 170–180 bpm for a specific number of beats or period of time. The atrial tachycardia/atrial fibrillation (AT/AF) diagnostic suite provided detailed historical data, allowing us to identify and evaluate therapy for improved management of patients. Atrial sensitivity was programed to 0.5 mV.
Patients were evaluated 15 days after pacemaker implantation to assess the pocket, the site of the surgical incision, and to adjust the programing of the pacemaker. Fifteen days later, the patients returned for reassessment (1 month after pacemaker implantation). Data were obtained from the pacemaker at 1 month post implant. Thereafter, patients were assessed every 6 months up to 3 years of follow-up. At each follow-up visit, we obtained a record (stored on a USB stick and then transferred to a computer) of the pacemaker memory data that had accumulated since the previous resetting of the memory. The onset of the first AF episode was also registered in each patient’s data record. Time to AF onset was defined as the number of days from baseline to the first recorded episode of AF lasting ≥30 s. Patients were censored due to death, loss to follow-up, or 18 months’ post-implant. According to our standard of care, all of the patients underwent echocardiography at baseline. The results are expressed as a mean and standard deviation for normally distributed data and as median with interquartile range otherwise. All statistical tests were two-sided. Comparisons between two-paired values were performed with the paired t-test in cases of a Gaussian distribution or by the Wilcoxon test otherwise. Comparisons between more than two-paired values were made by repeated-measures analysis of variance or by Kruskal–Wallis analysis of variance as appropriate, complemented by a post-hoc test. Categorical variables were compared with Fisher’s exact test. A P-value <0.05 was considered significant. Correlations between two variables were performed by Pearson’s chi-square test in case of a Gaussian distribution and with the Spearman correlation test otherwise. The Kaplan–Meier method was used to describe the freedom from AF events over time, and differences between groups were assessed by the log-rank test. All statistical analyses were performed using the program Graphpad Prism v 7.0 (Graphpad Software, La Jolla, CA, USA).
We screened 634 patients who received a DDD pacemaker to treat third- or second-degree Mobitz II AV block for preliminary inclusion. Among these, only 81 patients had presented stroke or TIA previously the implant. The baseline features of patients in both groups are shown in Table 1. From these 81 patients, sixty-four (79%) subjects had silent AF detected (Figure 1) in a mean time of 11.8 months of follow up. In conclusion, the present investigation shows that pacemaker can be a useful tool to detect silent AF events in patients with previous stroke or TIA without an identifiable before the pacemaker implant.
Table 1. Baseline features of patients
Baseline features of patients |
N |
81 |
Age, years |
84.7 ± 9.1 |
Body mass index, Kg/m2 |
29.8 ± 2.3 |
Male gender |
51 (63%) |
White ethnicity |
45 (56%) |
Stroke/transient ischaemic attack |
81 (100%) |
Hypertension |
70 (86%) |
Coronary artery disease |
50 (62%) |
Type 2 Diabetes Mellitus |
62 (77%) |
Left ventricular dysfunction |
44 (54%) |
Mean 24-hours systolic/diastolic ABPM, mmHg |
141.0 ± 6.5/92.4 ± 4.8 |
Mean left ventricular ejection fraction, % |
44.3 ± 5.9 |
Creatinine, mg/dL |
1.90 ± 0.23 |
eGFR, mL/min/1.73m² (CKD-EPI) |
32.0 ± 6.5 |
Ventricular Pacing (%) |
97.4 ± 12.3 |
Values are presented as mean ± SD or %. ABPM, ambulatory blood pressure measurements; eGFR, estimated glomerular filtration rate; N, the number of patients. Patients with coronary artery disease had undergone coronary angiography or computed tomography angiography of coronary arteries at some time in their life. This indicated that they had some degree of obstruction in the coronary arteries, but this did not cause ischemia.
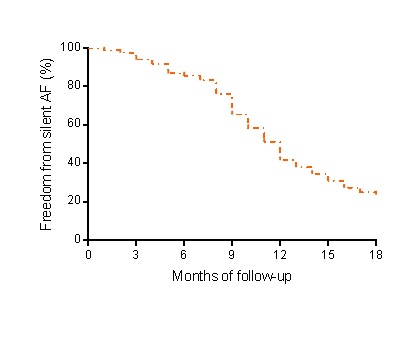
Figure 1. Kaplan–Meier analysis for silent atrial fibrillation events during 18 months of follow-up, in patients with dual chamber pacemaker that suffered a stroke or transient ischemic attack before.
None declared.
The authors are grateful to all study participants. The authors also thank Pacemed for contributing to the development of this study and for providing technical support.
- Kirchhof P, Auricchio A, Bax J, Crijns H, Camm J, et al. (2007) Outcome parameters for trials in atrial fibrillation: executive summary. Eur Heart J 28: 2803-2817. [Crossref]
- Hobbs FD, Fitzmaurice DA, Mant J, Murray E, Jowett S, et al. (2005) A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study. Health Technol Assess 9: iii–iv, ix–x, 1–74. [Crossref]
- Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, et al. (2010) Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 31: 2369-2429. [Crossref]

