Visceral obesity is one of the main causes of metabolic syndrome,as well as a risk factor for cerebro-/cardiovascular diseases. Therefore, in addition to lifestyle correction, effective drug therapy should be established. In this study, we administered ezetimibe to dyslipidemia patients with metabolic syndrome, and compared its effects on serum lipids and visceral obesity with those of statins.We randomly assigned outpatients to receive ezetimibe (EZ) or atorvastatin (AT), and examined changes in the serum levels of lipids and umbilical visceral fat area for 24 weeks.During the study period, 15 patients each were registered to receive EZ or AT. In the EZ group, the total cholesterol (TC)(p<0.01), LDL-cholesterol (LDL-C)(p<0.01), triglyceride (TG)(p<0.01), and remnant-like lipoprotein cholesterol (RLP-C)(p<0.01) levels significantly decreased. There was also a significant reduction in theumbilical visceral fat area (from 158.1 ± 11.0 to 156.2 ± 11.5 cm2) (p=0.01). In the AT group, there were significant decreases in the TC (p<0.05), LDL-C (p<0.01), and RLP-C (p<0.01) levels, but there were no significant changes in the umbilical visceral fat area.EZ may become a useful treatment option for dyslipidemia patients with obesity or metabolic syndrome.
hyperlipidemia, visceral fat, obesity, metabolic syndrome, ezetimibe
With lifestyle changes such as overeating and lack of exercise, the number of obese patients has markedly increased. In Japan, obese patients have been estimated to account for more than 30 and 20% of adult males and females, respectively [1]. Obesity, especially visceral obesity, is one of the main causes of metabolic syndrome. It causes dyslipidemia, type II diabetes, and hypertension, finally becoming a risk factor for arteriosclerotic disorders [2]. Therefore, obesity control is important.
Basic treatment for obesity is weight control by diet/exercise therapies, such as a decrease in energy intake and an increase in energy consumption. However, it is difficult to continue this treatment over a long period in many cases. Effective drug therapy should be established.
Ezetimibe selectively inhibits dietary/biliary cholesterol absorption by acting on a small intestinal cholesterol transporter, Niemann-Pick C1 like [1,3,4], thus, reducing the LDL-cholesterol (LDL-C) level by approximately 20% and improving hypertriglyceridemia and hypo-HDL-cholesterolemia [5]. In addition, it improves insulin resistance [6,7] and the vascular endothelial function [8] by correcting the postprandial disturbance of lipid metabolism; manifold effects are obtained.
Recent studies reported that ezetimibedecreased the visceral fat level in dyslipidemia patients with metabolic syndrome or non-alcoholic hepatopathy [9,10], but a control group was not established in these studies; there is a limitation.
In this study, we compared the influence of ezetimibe on visceral obesity with that of a statin, as a control drug, in dyslipidemia outpatients with obesity or metabolic syndrome.
The subjects were adults with a body mass index (BMI) of 25 kg/m2 or more, or meeting diagnostic criteria for metabolic syndrome in Japan [11], who consulted the outpatient clinic of our hospital between June 2011 and October 2011, showing an LDL-C level of 140 mg/dL or more despite diet/exercise therapies for 12 weeks or more.
They were randomly assigned to receive 10 mg of ezetimibe once a day (EZ group) or 10 mg of atorvastatin once a day (AT group). Each drug was orally administered for 24 weeks. During the study period, diet/exercise therapies were continued, and the introduction of other serum lipid-reducing drugs was avoided. We excluded patients taking drugs for diabetes or other serum lipid-reducing drugs, those with a history of hypersensitivity to ezetimibe or atorvastatin, those with severe liver/kidney diseases, and those who were not considered to be eligible.
We evaluated changes in the body weight, BMI, serum lipid levels, glucose metabolism, umbilical abdominal circumference, and umbilical visceral fat area after administration.
As serum lipid levels, the total cholesterol (TC), LDL-C, triglyceride (TG), HDL-cholesterol (HDL-C), and remnant-like particle cholesterol (RLP-C) levels were measured. Concerning glucose metabolism, the fasting blood glucose (FPG) level and HbA1c were determined. The umbilical visceral fat area on CT images obtained using a Pronto-XE system (Hitachi Medico) was analyzed using attached software.
To examine the additional effects of ezetimibe on statins, 10 mg of ezetimibe was additionally administered for 24 weeks to patients with an LDL-C level of >180 mg/dL despite the administration of atorvastatin at 10 mg. Similar items were investigated.
For statistical analysis, R version 2.13.0 software (http://www.r-project.org) was used. The results are expressed as the mean standard ± deviation. Concerning items in which a normal distribution was confirmed before and after administration, the paired or unpaired t-test was used. Concerning items in which there was no normal distribution, Wilcoxon’s signed rank test or MannWhitney’s U test was used. The category data were compared using Fisher’s direct probability method. A P-value of 0.05 was regarded as significant.
After the purpose and contents of this study were explained to the subjects, written informed consent regarding participation in this study was obtained.
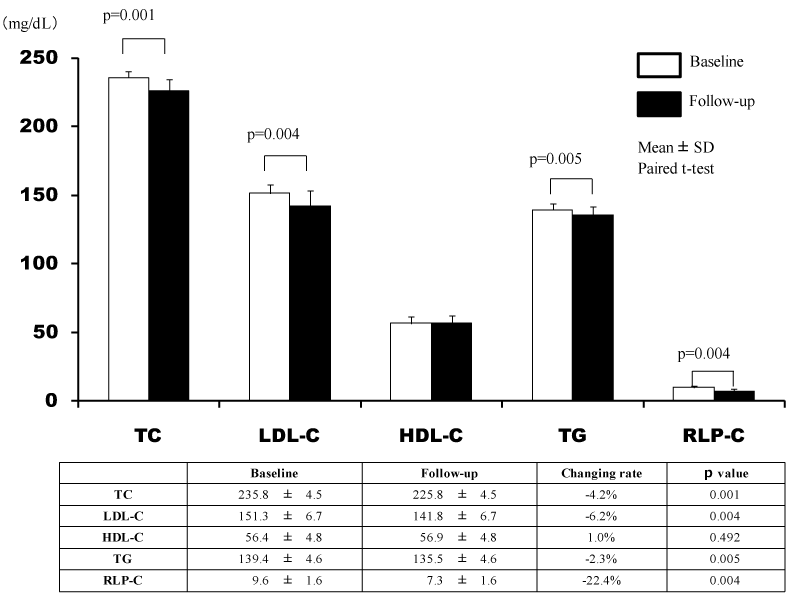
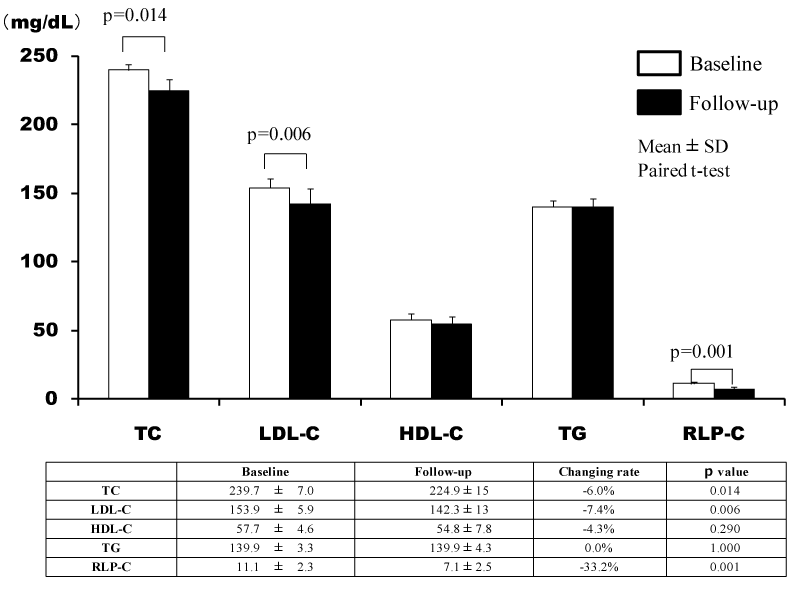
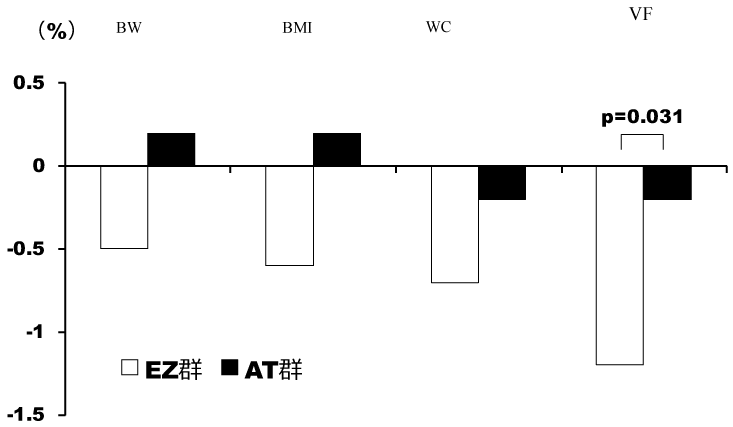
During the study period, 30 patients were registered, and randomly divided into 2 groups (EZ group: n=15, AT group: n=15). There were no significant differences in background factors, excluding HbA1c, between the two groups (Table 1). In the two groups, the TC, LDL-C, and RLP-C levels significantly decreased after 24 weeks, but there was a significant decrease in the TG level only in the EZ group (Figures 1 and 2). In the EZ group, the umbilical visceral fat area significantly decreased from 158.1 ± 11.0 to 156.2 ± 11.5 cm2 (p=0.010). However, in the AT group, there were no significant changes (from 152.9 ± 7.2 to 152.6 ± 7.3 cm2) (p=0.423). In the EZ group, there was a significant decrease in the umbilical visceral fat area in comparison with the AT group (p=0.031) (Table 2, Figure 3).
Table 1. Background of patients
|
EZ group(n=15) |
AT group(n=15) |
P value |
Gender (male/female) |
5/10 |
5/10 |
1.0001 |
Age (years) |
65.1 ± 6.4 |
64.2 ± 8.9 |
0.7442 |
Body weight (kg) |
69.1 ± 10.8 |
69.7 ± 10.8 |
0.8802 |
Body Mass Index (kg/m2) |
27.4 ± 2.5 |
27.5 ± 2.2 |
0.8642 |
Waist circumference (cm) |
88.0 ± 2.8 |
87.9 ± 2.2 |
0.9422 |
Visceral fat (cm2) |
158.1 ± 11.0 |
152.9 ± 7.2 |
0.1372 |
Total Cholesterol (mg/dL) |
235.8 ± 4.5 |
239.7 ± 7.0 |
0.0822 |
LDL-Cholesterol (mg/dL) |
151.3 ± 6.9 |
153.9 ± 5.9 |
0.2802 |
HDL-Cholesterol (mg/dL) |
56.4 ± 4.8 |
57.7 ± 4.6 |
0.4512 |
Ttiglycerides (mg/dL) |
139.4 ± 4.6 |
139.9 ± 3.3 |
0.7202 |
RLP-Cholesterol (mg/dL) |
9.6 ± 1.6 |
11.1 ± 2.3 |
0.0572 |
Fasting plasma glucose (mg/dL) |
92.3 ± 7.2 |
89.5 ± 4.0 |
0.1982 |
HbA1c (%) |
5.2 ± 0.1 |
5.0 ± 0.1 |
0.0192 |
Systlic blood pressure (mmHg) |
131.2 ± 3.9 |
129.7 ± 2.6 |
0.2372 |
Diastolic blood pressure (mmHg) |
66.3 ± 5.7 |
68.8 ± 4.5 |
0.1862 |
Data are presented as Mean ± SD: 1Fisher’s exact test, 2Unpaired t-test
Table 2. Significant decrease in the umbilical visceral fat area in comparison with the AT group
EZ group |
Before |
24 weeks after |
Changing rate % |
P Value |
BW kg |
69.7 ± 10.8 |
68.6 ± 9.6 |
-0.5 ± 2.0 |
0.229 |
BMI (kg/m2) |
27.4 ± 2.5 |
27.2 ± 2.1 |
-0.6 ± 2.0 |
0.248 |
WC cm |
88.0 ± 2.8 |
87.4 ± 2.3 |
-0.7 ± 1.2 |
0.057 |
VF cm2 |
158.1 ± 11.0 |
156.2 ± 11.5 |
-1.2 ± 1.6 |
0.010 |
FBS mg/dl |
92.3 ± 7.2 |
90.8 ± 46.9 |
-2.1 ± 6.5 |
0.186 |
Hb A1c (%) |
5.2 ± 0.1 |
5.1 ± 0.3 |
-0.2 ± 2.5 |
0.220 |
Systolic (mmHg) |
131.2 ± 3.9 |
131.5 ± 2.8 |
0.2 ± 2.4 |
0.751 |
Diastolic (mmHg) |
66.3 ± 5.7 |
66.9 ± 4.7 |
1.4 ± 8.1 |
0.635 |
AT group |
Before |
24 weeks after |
Changing rate % |
P Value |
BW kg |
69.7 ± 10.8 |
69.8 ± 10.2 |
0.2 ± 2.0 |
0.860 |
BMI (kg/m2) |
27.5 ± 2.2 |
27.6 ± 2.1 |
0.2 ± 2.0 |
0.736 |
WC cm |
80.9 ± 2.2 |
87.7 ± 2.0 |
-0.2 ± 1.1 |
0.424 |
VF cm2 |
152.9 ± 7.2 |
152.6 ± 7.3 |
-0.2 ± 0.7 |
0.423 |
FBS mg/dl |
89.5 ± 4.0 |
88.4 ± 3.4 |
-0.6 ± 2.4 |
0.324 |
Hb A1c (%) |
5.0 ± 0.1 |
5.0 ± 0.1 |
-0.2 ± 2.5 |
0.313 |
Systolic (mmHg) |
129.7 ± 2.6 |
128.7 ± 2.4 |
-0.8 ± 1.7 |
0.088 |
Diastolic (mmHg) |
68.8 ± 4.5 |
67.1 ± 4.8 |
-2.3 ± 8.1 |
0.238 |
Mean ± SD, Paired t-test
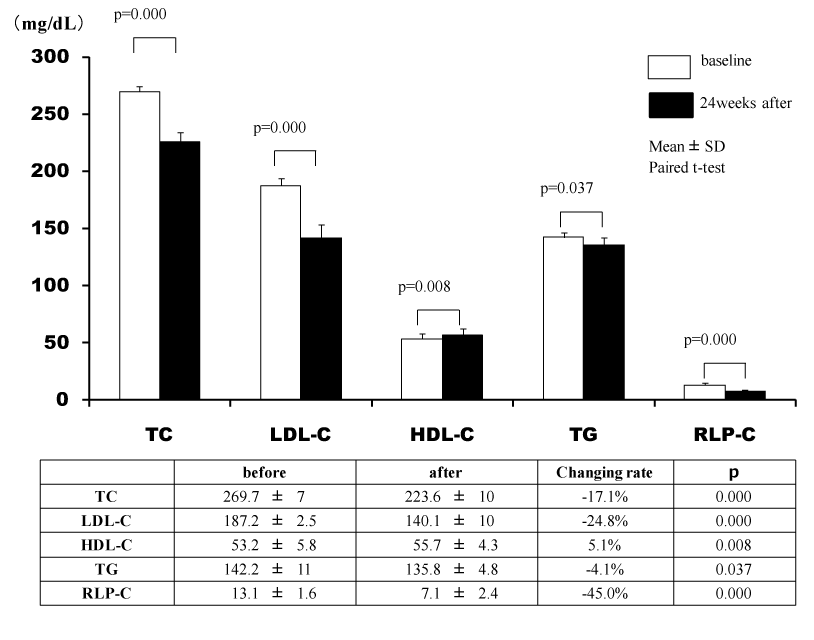
Table 3. Additional effects of ezetimibe on statins
|
Before |
24 weeks after |
Changing rate(%) |
p |
BW(kg) |
70.0 ± 10.9 |
69.4 ± 9.7 |
-0.7 ± 2.0 |
0.180 |
BMI(kg/m2) |
27.1 ± 1.9 |
26.9 ± 1.6 |
-0.7 ± 2.0 |
0.222 |
WC(cm) |
87.4 ± 2.4 |
85.6 ± 2.3 |
-2.1 ± 2.1 |
0.003 |
VF(cm2) |
147.2 ± 10.9 |
144.9 ± 11.0 |
-1.5 ± 2.0 |
0.019 |
FBS(mg/dL) |
84.4 ± 5.9 |
85.7 ± 6.3 |
1.5 ± 4.2 |
0.200 |
HbA1c (%) |
5.0 ± 0.1 |
5.0 ± 0.1 |
0.0 ± 4.0 |
1.000 |
systolic(mmHg) |
128.0 ± 2.8 |
125.7 ± 5.5 |
-1.8 ± 2.0 |
0.128 |
diastolic(mmHg) |
65.7 ± 4.2 |
67.4 ± 4.4 |
-3.0 ± 10.2 |
0.379 |
The lipid profiles of 13 patients in whom the additional effects of ezetimibe on statins were investigated were markedly improved. The umbilical visceral fat area significantly decreased from 147.2 ± 10.9 to 144.9 ± 11.0 cm2 (p=0.019). In addition, there was a decrease in the umbilical abdominal circumference (p=0.003) (Figure 4, Table 3).
During the study period, there were no clinically significant adverse events, including abnormal laboratory data, in any patient.
This study showed that ezetimibe reduced visceral obesity in dyslipidemia patients with obesity or metabolic syndrome. Theezetimibe-related decrease in the umbilical visceral fat area was slight, but significant. There was a decrease in 22 of 28 patients treated with ezetimibe (no change: 3 patients, increase: 3 patients).
There was no correlation between changes in the umbilical visceral fat area and lipids. Although the mechanism by which ezetimibe decreases the visceral fat area is unclear, no study has reported the usefulness of lipid-reducing drugs other than ezetimibe for visceral fat reduction. Therefore, the improvement of postprandial metabolism, including the restricted cholesterol absorption-related reduction of postprandial hyperlipidemia, may have been involved. Hiramitsu et al. [12] investigated the effects of ezetimibein postprandial hyperlipidemia volunteers on a high-fat/-glucose test diet, and reported that, after 4-week ezetimibe therapy, the TG level significantly decreased 4 and 6 hours after meals, and that there were also significant decreases in the blood glucose and insulin levels after meals, suggesting postprandial lipid/glucose metabolism-improving effects. Takase et al. [13] examined the effects of ezetimibe on visceral obesity in metabolic syndrome patients with an LDL-C level of >120 mg/dL, establishing a non-ezetimibe-treated group as a control group. They indicated that ezetimibe administration decreased the visceral fat area, improving insulin resistance, increasing the adiponectin level, and decreasing the RLP-C and oxidized LDL-C levels. They also reported that there was a correlation between an ezetimibe-related decrease in the visceral fat area and an increase in the adiponectin level.
However, this study involved patients without complications, such as diabetes and hypertension, other than hypercholesterolemia, and there were no significant changes in items such as the fasting blood glucose level and HbA1c after ezetimibe administration. On the other hand, the rate of decrease in the LDL-C level was 2 to 3% in the EZ and AT groups, being lower than previously reported. However, new patients without complications were enrolled, and adherence to drug therapy for 24 weeks may have been poor. In patients to whom ezetimibe was additionally administered after statin treatment, the LDL-C level decreased by approximately 25%; the percentage was similar to those previously reported [14].
Metabolic syndrome is considered to be a high risk factor for coronary disease, independent of hyper-LDL-cholesterolemia. When the two diseases are concomitantly present, the risk of coronary disease may increase. Therefore, intervention for the two diseases is necessary.
Ezetimibe reduces the serum levels of lipids in dyslipidemia patients with obesity or metabolic syndrome, and may be effective for visceral obesity; therefore, this drug may become a useful treatment option.
2021 Copyright OAT. All rights reserv
- Prospective Studies Collaboration, Whitlock G, Lewington S, Sherliker P, Clarke R, et al. (2009) Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 373: 1083-1096. [Crossref]
- Garcia-Calvo M, Lisnock J, Bull HG, Hawes BE, Burnett DA, et al. (2005) The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1). Proc Natl Acad Sci USA 102: 8132-8137. [Crossref]
- Altmann SW, Davis HR Jr, Zhu LJ, Yao X, Hoos LM, et al. (2004) Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science 303: 1201-1204. [Crossref]
- Saito Y, Yamada N, Nakatani N (2007) Phase ? clinical study of Ezetimibe- Double-blind comparative study with cholestimide-. J Clin Ther Med 23: 493-522.
- Hiramitsu S, Ishiguro Y, Matsuyama H, Yamada K, Kato K, et al. (2010) The effects of ezetimibe on surrogate markers of cholesterol absorption and synthesis in Japanese patients with dyslipidemia. J Atheroscler Thromb 17: 106-114. [Crossref]
- Yagi S, Akaike M, Aihara K, Iwase T, Ishikawa K, et al. (2010) Ezetimibe ameliorates metabolic disorders and microalbuminuria in patients with hypercholesterolemia. J Atheroscler Thromb 17: 173-180. [Crossref]
- Yunoki K, Nakamura K, Miyoshi T, Enko K, Kohno K, et al. (2011) Ezetimibe improves postprandial hyperlipemia and its induced endothelial dysfunction. Atherosclerosis 217: 486-491. [Crossref]
- Takase H, Dohi Y, Okado T, Hashimoto T, Goto Y, et al. (2010) The effects of ezetimibe on visceral obesity in patients with metabolic syndrome-Investigation by abdominal computed tomography. Prog Med 30: 2451-2456. [Crossref]
- Park H, Shima T, Yamaguchi K, Mitsuyoshi H, Minami M, et al. (2011) Efficacy of long-term ezetimibe therapy in patients with nonalcoholic fatty liver disease. J Gastroenterol 46: 101-107. [Crossref]
- Matsuzawa Y (2005) Metabolic syndrome –definition and diagnostic criteria in Japan. J Jpn Soc Int Med 94: 794-809. [Crossref]
- Hiramitsu S, Miyagishima K, Ishii J, Matsui S, Naruse H, et al. (2012) Effect of ezetimibe on lipid and glucose metabolism after a fat and glucose load. J Cardiol 60: 395-400. [Crossref]
- Takase H, Dohi Y, Okado T, Hashimoto T, Goto Y, et al. (2012) Effects of ezetimibe on visceral fat in the metabolic syndrome: a randomised controlled study. Eur J Clin Invest 42: 1287-1294. [Crossref]
- Ballantyne CM, Houri J, Notarbartolo A, Melani L, Lipka LJ, et al. (2003) Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation 107: 2409-2415. [Crossref]
- Pearson TA, Denke MA, McBride PE, Battisti WP, Brady WE, et al. (2005) A community-based, randomized trial of ezetimibe added to statin therapy to attain NCEP ATP III goals for LDL cholesterol in hypercholesterolemic patients: the ezetimibe add-on to statin for effectiveness (EASE) trial. Mayo Clin Proc 80: 587-595. [Crossref]




