Previously, we reported that the acute rise in invasive systolic blood pressure (BP) associated with atrial fibrillation (AF) occurrence during renal nerve stimulation (RNS) is an important symbol of the locality of the renal nerves and propose the significance of renal sympathetic activity in the mechanisms of hypertension and AF triggers [1] in subjects with preserved renal function. However, we do not know how its function in patients with chronic kidney disease (CKD), more specifically in its initial stage. Recently, Professor Esler’s group proposed that endurance training influences many factors which may mediate a reduction in sympathetic activity [2]. Endurance exercise training clearly lowers sympathetic activity in sympatho-excitatory disease states [3,4] and is well-tolerated by patients with CKD [5,6]. BP control is a central component of the management of patients with renal impairment. Altering sympathetic activity is one strategy to reduce BP. Indeed, there is growing appreciation for the role of the sympathetic system in this setting, with recent studies demonstrating the satisfactory effects of reducing sympathetic activity on BP [7,8]. The effect of endurance training on renal sympathetic nerve activity has been measured in healthy humans (Meredith et al., 1991), but not in humans with CKD. Thus, proofs of principle studies are required. Thereafter, identifying the ideal patient population should be considered, as most exercise training studies in patients with CKD include only the healthiest of patients, which may limit the generalizability of the findings [9]. Optimization of standard training principles, exercise frequency, intensity, time and type may all influence physiological adaptations and thus need to be studied. The duration of previous studies in clinical populations has typically been 4 months of training at a moderate intensity, 3 days/week while studies in healthy populations have been considerably shorter in duration [10]. Longer duration interventions (≥4months) are more effective in lowering sympathetic activity in other clinical populations, thus Professor Esler’s group recommend adopting a similar approach in patients with CKD2, because it was previously proved in healthy individuals that intense endurance training has a more pronounced effect on autonomic balance [11], which may be beneficial to patients with high sympathetic tone.
Based on these aforementioned concepts, our group believes that in patients with controlled hypertension, paroxysmal AF, and mild CKD the mapping of the main trunk of the renal arteries using a 3D mapping system and performing RNS in some zones, divided per quadrants, can provoke an increase in invasive BP concomitantly with induction of paroxysmal AF episodes, presenting themselves different in sedentary and physical active well trained patients.
This transversal study involved 20 patients with controlled hypertension, a history of symptomatic paroxysmal AF, and mild CKD, divided into 2 groups: 10 sedentary patients and 10 active patients. The study was piloted in agreement with the Helsinki declaration and approved by the ethics committee of our institution. All patients signed the informed consent term before inclusion. This study was conducted at the Hospital e Clínica São Gonçalo, Rio de Janeiro, Brazil. Patients were recruited from June to December 2016 from the Arrhythmias and Artificial Cardiac Pacing Service of the same hospital. Patients with the combination of the following criteria were consecutively enrolled: (i) mean 24-hour systolic ambulatory blood pressure measurements (ABPM) of ≥100 mmHg <130 mmHg, and mean 24-hour diastolic ambulatory blood pressure <80 mmHg; (ii) essential hypertension for >1 year; (iii) a physically normal heart with an ejection fraction of >50% as measured by echocardiography (Simpson’s method); (iv) Paroxysmal AF with one monthly episode (Paroxysmal AF was defined as AF episodes lasting <7 days with spontaneous termination) referred PVI; (v) no use of antiarrhythmic drug; (vi) age of 18 to 80 years, (vii) estimated glomerular filtration rate (eGFR) >60 mL/min/1.73 m2 estimated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [12] (with microalbuminuria), (viii) classified as sedentary or physically active well trained patients (subjects that practice at least 60 minutes of run by day, 4 times during the week, at least during the previously 6 months), (ix) and the capacity to read, comprehend, and sign the informed consent form and attend the clinical tests. The patients that presented any of the subsequent criteria were excluded: (i) pregnancy; (ii) valvular disease with significant adverse sequelae; (iii) unstable angina, myocardial infarction, transient ischemic attack or stroke within the 6 months before the procedure; (iv) renovascular abnormalities; (v) psychiatric disease; (vi) allergy to ionic contrast medium; (vii) a known addiction to drugs or alcohol that affects the intellect; (viii) congestive heart failure presenting functional class II to IV symptoms according to New York Heart Association; (ix) a transverse left atrial diameter (LAD) >45 mm on transthoracic echocardiography; (x) or a previous AF ablation procedure.
The 40 renal arteries from the 20 subjects were divided into two subgroups (20 left renal arteries and 20 right renal arteries). The aim of this study was evaluated if RNS can provoke an increase in invasive BP concomitantly with induction of paroxysmal AF episodes in mild CKD patients and if there was any difference between sedentary and physically active patients. The procedures were performed in the catheterization laboratory with direct visualization using fluoroscopy and radiopaque contrast and, we also used the three-dimensional mapping system (EnSite Velocity; St. Jude Medical, St. Paul, Minnesota, USA) to anatomically construct the renal arteries and aorta and perform RNS in the selected sites. The patients were pretreated with diazepam or midazolam under the supervision of an anesthesiologist. Catheterization of the femoral artery by the standard Seldinger technique was performed after subcutaneous injection of a local anesthetic in the inguinal site. An 8-Fr valved sheath (St. Jude Medical, St. Paul, Minnesota, USA) was placed into this artery, and unfractionated heparin was managed as an intravenous bolus, targeting an activated coagulation time of >250 s in the first 10 min. During the procedure, the target activated coagulation time ranged from 250 to 350 s. An aortography and selective renal arteriographies were obtained with an RDC catheter to visualize the aorta and renal arteries. Through the RDC catheter a conventional dirigible quadripolar catheter Livewire™ (St. Jude Medical, St. Paul, Minnesota, USA), with a tip electrode of 2 mm, and other electrodes of 1 mm was introduced into the renal artery, under fluoroscopic guidance. Unipolar stimulation was performed from the tip of the catheter in each quadrant and segment of the renal arteries (Figure 1A) [1] based on 3D mapping reconstruction. The cycle length was set at 200 ms (300 ppm), pacing using the maximum-output voltage at 20 V, current of 8 mA, using a pulse amplitude of 2 ms, during 90 s. The EP-Tracer system (Schwarzer Cardiotek GmbH, Im Zukunftspark 3, 74076 Heilbronn, Germany) was used to record and monitor the rhythm changes throughout the procedure. And the DX2021 monitor system (Dixtal Biomédica Indústria e Comércio LTDA., SP, Brazil) was used to register the changes in invasive systolic BP during the procedure. After the stimulation we waited for the BP to return to baseline values and when AF event occurred together we also waited for the rhythm return to the sinus rhythm, what happened spontaneously after stopping the RNS, and before proceeding to the next stimulation site. The patients remained hospitalized in the ward for 24 h after the procedure.
The results are expressed as a mean and standard deviation for normally distributed data and as median with interquartile range otherwise. All statistical tests were two-sided. Comparisons between two-paired values were performed with the paired t-test in cases of a Gaussian distribution and by the Wilcoxon test otherwise. Comparisons between more than two-paired values were made by repeated-measures analysis of variance or by Kruskal–Wallis analysis of variance as appropriate, complemented by a post-hoc test. Categorical variables were compared with Fisher’s exact test. A P-value <0.05 was considered significant. Correlations between two variables were performed by Pearson’s chi-square test in case of a Gaussian distribution and with the Spearman correlation test otherwise. All statistical analyses were performed using the program Graphpad Prism v 7.0 (Graphpad Software, La Jolla, CA, USA).
The general features of both groups of patients are listed in Table 1. The correlation between the variation (∆) in invasive systolic BP and AF occurrence in each quadrant of the left and right renal arteries by Pearson and Spearman methods during the RNS for sedentary and physically active patients is demonstrated in Table 2. According to Figure 1B and 1C, we can observe which areas are most susceptible to increase of the invasive systolic BP concomitantly with AF occurrence in sedentary and physically active subjects, respectively. Sedentary patients presented area under the roc curve (AUC)= 0.9962/0.9998, 95% confidence interval (CI)=0.9991 to 0.9997/0.9998 to 0.9999 , P value <0.0001/<0.0001, sensitivity= 97% / 100%, specificity= 99% / 99%, and the cutoff point of ∆ invasive systolic blood pressure to trigger AF during RNS >27.5 mmHg/ >27.5 mmHg, from the left and right renal arteries, respectively. However, the physically active subjects showed the AUC= 0.9989/0.9999, 95% CI=0.9995 to 0.9998/0.9996 to 0.9999, P value <0.0001/<0.0001, sensitivity= 100% / 100%, specificity= 99% / 100%, and the cutoff point of ∆ invasive systolic blood pressure to trigger AF during RNS >22.5 mmHg/ >22.5 mmHg, from the left and right renal arteries, respectively.
Table 1. Baseline features.
Parameters |
Sedentary patients with CKD 2 |
Active patients with CKD 2 |
P value |
N |
10 |
10 |
--- |
Age, years |
53.2±8.7 |
55.0±10.2 |
0.6762 |
Body mass index, kg/m2 |
28.5±2.1 |
26.2±1.5 |
0.0114 |
Male gender (%) |
8 (80%) |
6 (60%) |
0.6285 |
White ethnicity (%) |
7 (70%) |
9 (90%) |
0.5820 |
Controlled hypertension |
10 (100%) |
10 (100%) |
1.0000 |
Type 2 Diabetes Mellitus (%) |
4 (40%) |
6 (60%) |
0.6563 |
Coronary artery disease |
0 (0%) |
0 (0%) |
1.0000 |
Previously paroxismal AF ablation |
10 (100%) |
10 (100%) |
1.0000 |
Referred to PVI |
10 (100%) |
10 (100%) |
1.0000 |
Mean24-hour systolic/diastolic ABPM, mmHg |
120.4±6.0/72.0±5.6 |
122.7±4.5/70.3±6.7 |
0.3450/0.5458 |
Creatinine, mg/dL |
1.10±0.12 |
1.00±0.08 |
0.0417 |
eGFR, mL/min/1.73 m2 (CKD-EPI) |
76.5±8.3 |
83.3±5.1 |
0.0405 |
ACR, mg/g |
82.5±14.1 |
70.1±10.3 |
0.0375 |
Antihypertensive agents |
|
ACEI/ARB |
10 (100%) |
10 (100%) |
1.0000 |
Diuretics |
10 (100%) |
10 (100%) |
1.0000 |
DHP Ca++ channel blockers |
10 (100%) |
10 (100%) |
1.0000 |
β-blockers |
8 (80%) |
5 (50%) |
0.3498 |
The values are presented as mean ± SD or %; ACEI, receptor inhibitor of angiotensin converting enzyme; ARB, angiotensin receptor blocker; DHP, dihydropyridine; N, number of patients.
Table 2. Data of the procedures (n=20 patients).
Sites |
20 Left Renal Arteries (n=320 sites) |
20 Right Renal Arteries (n=320 Sites) |
Sites where paroxysmal AF occurred during RNS, n (%) |
∆ mean invasive systolic BP during RNS, mmHg |
Sites where paroxysmal AF occurred during RNS, n (%) |
∆ mean invasive systolic BP during RNS, mmHg |
RNS per quadrant |
Sedentary
10 LRA
(n=160) |
Active
10 LRA
(n=160) |
Sedentary
10 LRA
(n=160) |
Active
10 LRA
(n=160) |
Sedentary
10 RRA
(n=160) |
Active
10 RRA
(n=160) |
Sedentary
10 RRA
(n=160) |
Active
10 RRA
(n=160) |
Quadrant1 - Ostium |
9 (90%) |
2 (20%)* |
30.3 |
19.2*** |
10 (100%) |
3 (30%)* |
30.9 |
16.5*** |
Quadrant2 - Ostium |
8 (80%) |
3 (30%) |
31.8 |
19.1*** |
8 (80%) |
0 (0%)** |
31.3 |
8.2*** |
Quadrant3 - Ostium |
7 (70%) |
0 (0%)* |
28.0 |
12.1*** |
8 (80%) |
3 (30%) |
28.3 |
12.2** |
Quadrant4 - Ostium |
9 (90%) |
0 (0%)** |
30.2 |
7.1*** |
0 (0%) |
2 (20%)** |
9.8 |
14.1 |
Quadrant1 - Proximal |
7 (70%) |
2 (20%) |
29.4 |
10.8*** |
8 (80%) |
0 (0%) |
35.2 |
9.9*** |
Quadrant2 - Proximal |
1 (1%) |
0 (0%) |
20.6 |
15.9 |
8 (80%) |
3 (30%) |
33.7 |
14.0*** |
Quadrant3 - Proximal |
0 (0%) |
0 (0%) |
10.7 |
5.8* |
0 (0%) |
0 (0%) |
9.3 |
8.0 |
Quadrant4 - Proximal |
0 (0%) |
0 (0%) |
9.6 |
7.4 |
0 (0%) |
2 (20%) |
11.4 |
12.2 |
Quadrant1 - Middle |
0 (0%) |
0 (0%) |
8.2 |
8.6 |
0 (0%) |
0 (0%) |
9.3 |
6.9 |
Quadrant2 - Middle |
0 (0%) |
4 (40%)** |
11.1 |
16.3 |
0 (0%) |
0 (0%) |
8.7 |
9.0 |
Quadrant3 - Middle |
8 (80%) |
0 (0%) |
28.7 |
9.2*** |
6 (60%) |
3 (30%) |
28.7 |
13.4** |
Quadrant4 - Middle |
0 (0%) |
0 (0%) |
4.7 |
6.0 |
0 (0%) |
0 (0%) |
4.7 |
6.5 |
Quadrant1 - Distal |
0 (0%) |
4 (40%) |
7.7 |
16.6* |
10 (100%) |
0 (0%)*** |
37.3 |
9.5*** |
Quadrant2 - Distal |
0 (0%) |
4 (40%) |
12.6 |
13.8 |
10 (100%) |
2 (20%)** |
34.8 |
10.2*** |
Quadrant3 - Distal |
10 (10%) |
0 (0%)*** |
33.5 |
2.9*** |
0 (0%) |
2 (20%) |
7.8 |
9.1 |
Quadrant4 - Distal |
9 (90%) |
0 (0%)** |
33.0 |
7.0*** |
0 (0%) |
0 (0%) |
12.2 |
8.4 |
Pearson correlation; 95% CI; P value |
Sedentary: r=0.9626; 95%CI: 0.8929 – 0.9872; P<0.0001 |
Sedentary: r=0.9752; 95%CI: 0.9281 – 0.9916; P<0.0001 |
Spearman correlation; 95% CI; P value |
Sedentary: r=0.9317; 95%CI: 0.8046 – 0.9772; P<0.0001 |
Sedentary: r=0.8733; 95%CI: 0.6569 – 0.9568; P<0.0001 |
Pearson correlation; 95% CI; P value |
Active: r=0.7171; 95%CI: 0.3435 – 0.8947; P=0.0018 |
Active: r=0.8466; 95%CI: 0.6047 – 0.9455; P<0.0001 |
Spearman correlation; 95% CI; P value |
Active: r=0.7319; 95%CI: 0.3567 – 0.9038; P=0.0019 |
Active: r=0.8379; 95%CI: 0.5746 – 0.9440; P=0.0001 |
AF, atrial fibrillation; BP, blood pressure; ∆, variation; LRA, left renal artery; RNS, renal nerve stimulation; RRA, right renal artery; 95%CI. 95% Confidence Interval; *P<0.05, **P<0.001, and ***P<0.0001 for comparisons between sedentary vs. active subjects during RNS of renal arteries at the same side of the body.
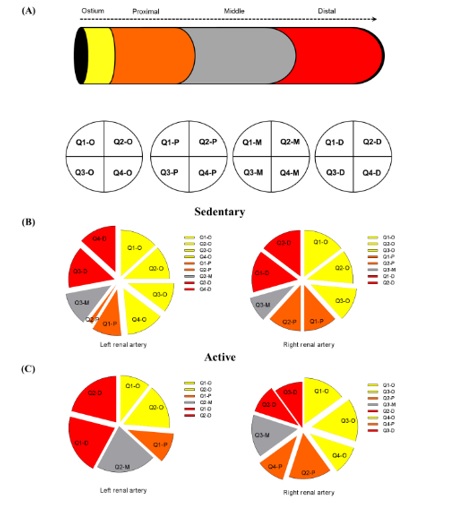
Figure 1. A) Schematic design of the right renal artery divided per segments: ostium, proximal, middle and distal parts, and each part sliced separated in four quadrants. In (B) sedentary and (C) physically active subjects: the most susceptible zones in the left and right renal arteries where there was increasing the invasive systolic blood pressure concomitantly with atrial fibrillation occurrence; D, distal; M, middle; O, ostium; P, proximal; Q, quadrant.
In conclusion, our study shows that the acute rise in invasive systolic BP associated to AF occurrence during RNS is a significant marker of the location of the renal nerves, creating a trail and suggesting the importance of renal sympathetic activity in the mechanisms of hypertension and AF triggers. During RNS higher levels of BP associated with AF trigger were targeted in sedentary patients in comparison to physically active patients, however, less AF episodes were observed in the last ones, possibly due to the practice of regular physical activity, leading to the reduction of the sympathetic over activity state that occurs in CKD.
None declared.
This study was funded by Pacemed (US $200,000), Rio de Janeiro, Brazil.
The authors are grateful to all participants included in this study. The authors also thank Pacemed for stimulating the development of this study and for providing technical support.
- Kiuchi MG and Chen S. (2016) Renal sympathetic stimulation in patients with controlled hypertension and paroxysmal atrial fibrillation. Int J Cardiol 224: 394-397. [Crossref]
- Howden EJ, Lawley JS, Esler M, Levine BD. (2016) The potential role of endurance training in altering renal sympathetic nerve activity in CKD? Auton Neurosci S1566-0702(16)30263-6. [Crossref]
- Carter JR, Ray CA (2015) Sympathetic neural adaptations to exercise training in humans. Auton Neurosci 188: 36-43. [Crossref]
- Haack KK, Zucker IH. (2015) Central mechanisms for exercise training-induced reduction in sympatho-excitation in chronic heart failure. Auton Neurosci 188: 44-50. [Crossref]
- Howden EJ, Coombes JS, Strand H, Douglas B, Campbell KL, et al. (2015) Exercise training in CKD: efficacy, adherence, and safety. Am J Kidney Dis 65: 583-591. [Crossref]
- Stray-Gundersen J, Howden EJ, Parsons DB, Thompson JR. (2016) Neither hematocrit normalization nor exercise training restores oxygen consumption to normal levels in haemodialysis patients. J Am Soc Nephrol 27: 3769-3779. [Crossref]
- Gamboa A, Figueroa R, Paranjape SY, Farley G, Diedrich A, et al. (2016) Autonomic blockade reverses endothelial dysfunction in obesity-associated hypertension. Hypertension 68: 1004-1010. [Crossref]
- Diedrich A, Jordan J, Tank J, Shannon JR, Robertson R, et al. (2003) The sympathetic nervous system in hypertension: assessment by blood pressure variability and ganglionic blockade J Hypertens 21: 1677-1686. [Crossref]
2021 Copyright OAT. All rights reserv
- Howden EJ, Fassett RG, Isbel NM, Coombes JS (2012) Exercise training in chronic kidney disease patients. Sports Med 42: 473-488. [Crossref]
- Carter JR, Ray CA (2015) Sympathetic neural adaptations to exercise training in humans. Auton Neurosci 188: 36-43. [Crossref]
- Iwasaki K, Zhang R, Zuckerman JH, Levine BD. (2003) Dose-response relationship of the cardiovascular adaptation to endurance training in healthy adults: how much training for what benefit? J Appl Physiol 95: 1575-1583. [Crossref]
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, et al. (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604-612. [Crossref]

