Abstract
Background: In osteoarthritis (OA) of the most common joint disease, inflammation is deeply involved in the cartilage degradation. Specifically, inflammatory cytokines like IL-1β are known to enhance production of matrix-degrading enzymes like matrix metalloproteinase (MMP) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS). Recently, soy peptides, generated by hydrolytic enzymatic digestion of soy proteins, have been found to show anti-inflammatory activity. Thereby we investigated whether soy peptides affected IL-1β-induced expression of matrix-degrading enzymes in human articular chondrocytes. Methods: Articular chondrocytes derived from 4 patients with bone fracture and 11 patients with OA were cultured with 0, 10, or 100 µM soy peptides for 4 hours. Then, the cells were stimulated with IL-1β for 24 hours. Non-stimulated cells were used as a control. RNA extracted from the cells was subjected to quantitative real time-PCR to assess amounts of mRNA for MMP-3 and ADAMTS-4. Results: Treatment with 100 µM soy peptides significantly suppressed mRNA levels of MMP-3 and ADAMTS-4 enhanced by the IL-1β stimulation (p=0.036 and p=0.005, respectively), although the treatment with 10 µM soy peptides did not show significant suppression (p=0.91 and p=0.20, respectively). In the 100 µM soy peptides treatment, the suppression of MMP-3 mRNA levels was correlated with that of ADAMTS-4 mRNA levels (R2=0.534). Conclusion: Soy peptides suppressed the IL-1β-induced expression of matrix-degrading enzymes in human articular chondrocytes. Soy peptides may have potential to ameliorate cartilage degradation in OA.
Key words
A disintegrin and metalloproteinase with thrombospondin motifs-4 (ADAMTS-4), Chondrocytes, IL-1β, Matrix metalloproteinase-3 (MMP-3), Osteoarthritis; Soy peptides
Introduction
Osteoarthritis (OA), the most common joint disease, is histologically characterized by degeneration of cartilage and formation of osteophytes, which lead to destruction of joints [1]. Many patients with OA suffer from pain and disability [1]. Thus, to suppress the symptoms of OA and prevent the progress of OA, novel agents should be developed.
Articular chondrocytes maintain the cartilage matrix by producing and degrading matrix proteins such as type II collagen and proteoglycans [2]. In OA, imbalance between production and degradation of the matrix protein leads to cartilage degradation [1]. Inflammation is deeply involved in the cartilage degradation in OA [1]. Inflammatory cytokines like IL-1β are known to be increased in synovial fluid of patients with OA [3]. In particular, IL-1β was reported to enhance secretion of matrix metalloproteinase (MMP)-1, -3, and -13 from chondrocytes that digest multiple types of collagens [4]. Furthermore, IL-1β was reported to enhance secretion of a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-4 and -5 from chondrocytes that digest aggrecan of a representative proteoglycan [5].
Soybeans contain a large amount of nutrients such as proteins, fats, and carbohydrates. Recently, novel biological roles of soybeans have been highlighted in addition to the roles as nutrients. Specifically, so-called soy peptides, generated by hydrolytic enzymatic digestion of soy proteins, have been reported to show various functions beneficial to health such as cholesterol-lowering properties, cognitive impairment-prevention, antiviral effects, and anti-inflammatory effects [6-12]. As to the anti-inflammatory effects, soy peptides were reported to suppress lipopolysaccharide (LPS)-induced production of cyclooxygenase-2 and prostaglandin E2 in a murine macrophage cell line [10]. Furthermore, soy peptides were reported to suppress LPS-induced production of pro-inflammatory cytokines such as IL-6 and IL-8 in human umbilical vein endothelial cells [11]. In vivo, soy-derived di- and tri-rich peptides were reported to alleviate colon and ileum inflammation in pigs with dextran sodium sulfate-induced colitis [12]. Therefore, soy peptides are expected to show anti-inflammatory effects also in human articular chondrocytes, which may lead to suppression of cartilage degradation in OA. However, effects of soy peptides on human articular chondrocytes have not been investigated to our knowledge. We here investigated whether soy peptides suppressed IL-1β-induced expression of matrix-degrading enzymes in human articular chondrocytes.
Materials and methods
Clinical samples and preparation of chondrocytes
Human articular cartilage samples were obtained from 4 patients with bone fracture (Normal, N1-4, 1 male; age 58 years, 3 female; mean age 78.0 years [range58-88]) at therapeutic surgery of total hip arthroplasty. Cartilage samples were also obtained from 11 patients with OA (OA1-11, 1 male; age 82 years, 10 female; mean age 78.7 years [range 66-86 years]) at therapeutic surgery of total knee arthroplasty.
The diagnosis of OA was made according to the Kellgren and Lawrence criteria [13]. Written informed consent was obtained from each of the patients and this study protocol was approved by the ethics committee of St. Marianna University School of Medicine. The study was performed in compliance with the World Medical Association Declaration of Helsinki.
For chondrocyte preparation, after careful removal of synovial tissue from cartilage samples, the cartilage was minced, washed, and treated with collagenase (Wako Pure Chemical Industries, Osaka, Japan). Isolated chondrocytes (Passage 0, P0) were washed and grown in monolayer culture in Dulbecco’s modified Eagle’s medium (DMEM, GIBCO, Carlsbad, CA) supplemented with 10% fetal calf serum (Wako), 100 units/ml penicillin, and 100 μg/ml streptomycin (Sigma-Aldrich, St. Louis, MO, USA) on type I collagen-coated culture dishes.
Treatment of chondrocytes with IL-1β and soy peptides
Soy peptides (Hinute-AM, FUJI OIL Co. Ltd., Osaka, Japan), which were mainly composed of di- and tri-peptides, were used [14].
The prepared chondrocytes (P1-P3, 1.0x107 cells/f100mm dish) were cultured with 0, 10, and 100 µM soy peptides for 4 hours. Then, the cells were treated with 1 µg/ml IL-1β alone (R&D, Minneapolis, MN, USA), 1 µg/ml IL-1β and 10 µM soy peptides, and 1 µg/ml IL-1β and 100 µM soy peptides for 24 hours. Non-treated cells were used as a control.
Evaluation of cell viability
Cell viability was evaluated by trypan blue staining. Chondrocytes, cultured with 0, 10, or 100 µM soy peptides for 24 hours, were harvested by treatment with 0.1% trypsin-EDTA (Sigma-Aldrich). The cell pellet, collected by centrifugation for 10 min at 1000×g, was stained with 0.5% trypan blue (Sigma-Aldrich). The number of the blue -stained (dead) cells and the total number of cells were counted using a haemocytometer under a microscopy.
Extraction and reverse transcription (RT) of RNA
Extraction of RNA from the chondrocyte samples and reverse-transcription of the RNA samples were performed using RNeasy® (Qiagen, Venlo, the Netherlands) and High Capacity cDNA Reverse Transcription Kits® (Life Technologies, Rockville, MD, USA), respectively.
Quantitative real time PCR
Real time PCR was performed using ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster city, CA, USA), according to the manufacturer’s instructions. To measure mRNA levels for MMP-3 and ADAMTS-4, 1 µg of total RNA-derived cDNA was mixed with TaqMan® Gene Expression Assays (Hs00968305_m1 and Hs00192708_m1, respectively, Applied Biosystems) and TaqMan® Gene Expression Master Mix (Applied Biosystems). Then the mixture was subjected to real time PCR. The thermal cycle condition was as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 60 s. To measure mRNA levels for GAPDH, 1 µg of total RNA-derived cDNA was mixed with 300 nM each of the forward and reverse primers and Power SYBR® Master Mix (Applied Biosystems). Nucleotide sequences of the primers for amplification of a GAPDH DNA fragment are as follows: for GAPDH: 5’-TGGTATGGTGGAAGGACTCA and 5’-ATGCCAGTGAGCTTCCCGTT. Real time PCR was performed under the same condition as above.
Statistical analysis
Statistical analysis was performed by IBM SPSS Statistics (Ver.22.0). Effects of soy peptides on IL-1β-induced matrix-degrading enzymes were analyzed by Wilcoxon signed-rank test (p<0.05).
Results
Effects of soy peptides on IL-1β-induced matrix-degrading enzymes in human articular chondrocytes
Firstly, we tested whether the concentrations of soy peptides used here showed cytotoxicity against human articular chondrocytes. As a result, we confirmed that up to 100 µM soy peptides did not affect viability of the chondrocytes (Figure 1A). We then investigated effects of the soy peptides on IL-1β-induced expression of MMP-3 and ADAMTS-4 in the chondrocytes. We cultured chondrocytes with or without the soy peptides for 4 hours and then stimulated with IL-1β. We then assessed mRNA levels of MMP-3 and ADAMTS-4.
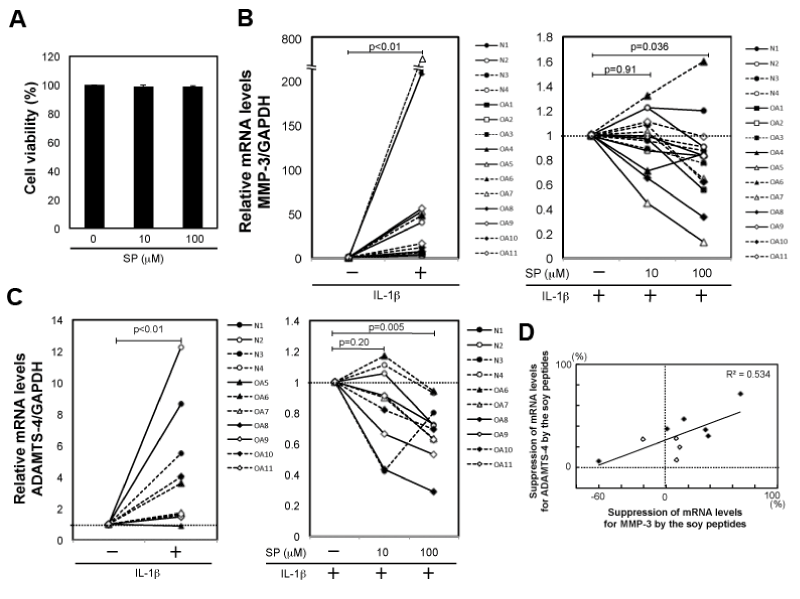
Figure 1. Effects of soy peptides on IL-1β-induced expression of MMP-3 and ADAMTS-4 in human articular chondrocytes. (A) Articular chondrocytes from 3 patients with OA (OA1-3) were cultured with 0, 10, and 100 µM soy peptides for 24 hours. The cells were subjected to trypan blue staining. The cell viability (%) was calculated using the following formula; (the number of unstained cells/the total number of cells)×100. SP, soy peptides. (B) Articular chondrocytes from 4 patients with bone fracture (N1-4) and 11 patients with OA (OA1-11) were cultured with 0, 10, and 100 µM soy peptides for 4 hours. Then, the cells were stimulated with IL-1β for 24 hours. Non-stimulated cells were used as a control. The mRNA levels for MMP-3 and GAPDH were assessed by RT-qPCR. The mRNA levels of MMP-3 were corrected using those of GAPDH. Left: The corrected mRNA level of MMP-3 was defined as 1.0 in each of the non-treated samples. Right: The corrected mRNA level of MMP-3 was defined as 1.0 in each of the IL-1β-treated samples. p-values were calculated by Wilcoxon signed-rank test using the values listed in Table 1. (C) Articular chondrocytes from 4 patients with bone fracture (N1-4) and 7 patients with OA (OA5-11) were treated similarly as described in (A). The mRNA levels of ADAMTS-4 were corrected using those of GAPDH. Left: The corrected mRNA level of ADAMTS-4 was defined as 1.0 in each of the non-treated samples. Right: The corrected mRNA level of ADAMTS-4 was defined as 1.0 in each of the IL-1β-treated samples. p-values were calculated by Wilcoxon signed-rank test using the values listed in Table 2. SP, soy peptides. (D) Suppression rates (%) of mRNA levels for MMP-3 and ADAMTS-4 in each of the chondrocyte samples (4 patients with bone fracture (N1-4) and 6 patients with OA (OA6-11)) were calculated by using the values listed in Table 1 and 2, respectively. A correlation diagram between the suppression of mRNA levels for MMP-3 and ADAMTS-4 in the treatment with 100 µM soy peptides treatment is shown. Coefficient of determination (R2) was calculated by regression analysis.
In the assessment of mRNA levels of MMP-3, we first confirmed that mRNA levels of MMP-3 were significantly increased by the IL-1β stimulation (p<0.01, Table 1 and Figure 1B, left panel). Of note, in each of the stimulated chondrocyte sample, the mRNA levels of MMP-3 were increased by the IL-1β stimulation. We then evaluated effects of the soy peptides on the IL-1β-induced expression of MMP-3 in chondrocytes. As a result, the treatment with 100 µM soy peptides significantly suppressed mRNA levels of MMP-3 enhanced by the IL-1β stimulation (p=0.036, Table 1 and Figure 1B, right panel), although the treatment with 10 µM soy peptides did not show significant suppression (p=0.91, Table 1 and Figure 1B, right panel).
Sample |
Control |
IL-1β |
IL-1β |
IL-1β |
no. |
+10µM HN-AM |
+100µM HN-AM |
N1
|
0.063
0 |
13.2 |
16.2 |
15.9 |
N2
N3
|
1.49 |
60.0 |
73.3 |
54.4 |
N3 |
2.13 |
13.5 |
13.0 |
11.9 |
N4
|
1.45 |
10.3 |
10.1 |
9.27
|
OA1
|
1.09 |
4.62 |
4.49 |
2.58 |
OA2 |
2.93 |
7.98 |
6.98 |
6.62 |
OA3 |
0.77 |
3.17 |
2.85 |
2.48 |
OA4 |
0.91 |
6.50 |
4.60 |
5.52 |
OA5 |
0.81 |
42.5 |
19.2 |
5.72 |
OA6 |
0.33 |
15.9 |
20.7 |
25.0 |
OA7 |
0.02 |
15.9 |
16.3 |
10.3 |
OA8 |
1.58 |
12.1 |
7.93 |
4.04 |
OA9
|
0.20 |
10.9 |
10.9 |
9.13 |
OA10 |
1.02 |
13.0 |
14.2 |
8.11 |
OA11 |
0.22 |
3.55 |
3.91 |
3.50 |
Average
|
1.00 |
15.5 |
15.0 |
11.6 |
STDEV |
0.82 |
15.4 |
17.1 |
13.2 |
p value |
- |
<0.01
|
0.91 |
0.036
|
(vs Control) |
(vs IL-1β) |
(vs IL-1β) |
Table 1. Effects of soy peptides on IL-1β-induced MMP-3 mRNA levels in human chondrocytes
SP, soy peptides
In the assessment of ADAMTS-4, we first confirmed that mRNA levels of ADAMTS-4 were significantly increased by IL-1β stimulation (p<0.01, Table 2 and Figure 1C, left panel). Ten out of the 11 chondrocyte samples up-regulated the mRNA levels of ADAMTS-4 by the IL-1β stimulation. We thus evaluated effects of the soy peptides on the IL-1β-induced expression of ADAMTS-4 using the 10 chondrocyte samples that positively responded to IL-1β. As a result, the treatment with 100 µM soy peptides significantly suppressed mRNA levels of ADAMTS-4 enhanced by the IL-1β stimulation (p=0.005, Table 2 and Figure 1C, right panel), although the treatment with 10 µM soy peptides did not show significant suppression (p=0.20, Table 2 and Figure 1C, right panel).
Table 2. Effects of soy peptides on IL-1β-induced ADAMTS-4 mRNA levels in human chondrocytes
Sample |
Control |
IL-1β |
IL-1β |
IL-1β |
no. |
+10µM HN-AM |
+100µM HN-AM |
N1
|
0.16 |
1.37 |
1.25 |
1.00 |
N2
N3
|
0.23 |
2.78 |
2.94 |
2.00 |
N3 |
0.13 |
0.74 |
0.31 |
0.59 |
N4
|
0.92 |
1.49 |
1.65 |
1.38 |
OA5 |
2.03 |
1.77 |
- |
- |
OA6 |
1.30 |
4.71 |
5.53 |
4.43 |
OA7 |
1.70 |
2.96 |
2.66 |
1.88 |
OA8 |
1.02 |
1.51 |
0.66 |
0.43 |
OA9
|
1.47 |
2.20 |
1.46 |
1.16 |
OA10 |
0.91 |
3.64 |
2.98 |
2.52 |
OA11 |
1.11 |
1.83 |
1.66 |
1.14 |
Average
|
1.00 |
2.27 |
2.11 |
1.65 |
STDEV |
0.63 |
1.16 |
1.50 |
1.17 |
p value |
- |
<0.01
|
0.20 |
0.005
|
2021 Copyright OAT. All rights reserv
(vs Control) |
(vs IL-1β) |
(vs IL-1β) |
SP, soy peptides
Finally, we investigated correlation of the suppressing effect of the soy peptides on the MMP-3 expression to that of ADAMTS-4 expression. As a result, in the treatment with 100 µM soy peptides, the suppression of MMP-3 mRNA levels was correlated with that of ADAMTS-4 mRNA levels (R2=0.534, Figure 1D). In addition, the suppressive effect of the soy peptides on the expression of MMP-3 and ADAMTS-4 tended to be higher in patients with OA than in those with bone fracture (Figure 1D).
Discussion
We here found that soy peptides suppressed IL-1β-induced production of MMP-3 and ADAMTS-4 in human articular chondrocytes.
The possible mechanisms for the actions of soy peptides are as follows: 1) soy peptides inhibit the input of IL-1β signal transduction by binding to IL-1β or IL-1β receptors, 2) soy peptides, taken up into chondrocytes, inhibit the intracellular IL-1β signal transduction, and 3) soy peptides bind to their unknown receptors and then block the IL-1β signal transduction.
On the first possible mechanism, IL-1β activates the signal transduction system by specific binding to IL-1β receptors. In the majority of the tested chondrocytes samples, the mRNA levels of MMP-3 of the IL-1β-stimulated chondrocytes group were suppressed by the treatment with 100 µM soy peptides (Figure 1B). However, in a small part of the tested chondrocytes samples, the mRNA levels of MMP-3 were increased by the soy peptides treatment (Figure 1B). If soy peptides bound to IL-1β or IL-1β receptors, the mRNA levels of MMP-3 would have been suppressed in all the tested chondrocytes samples. Thus, it would be unlikely that soy peptides inhibit the binding between IL-1β and IL-1β receptors.
On the second possible mechanism, we showed that suppression of mRNA levels for MMP-3 were well correlated with that of mRNA levels for ADAMTS-4 in the soy peptides-treated chondrocyte samples (R2=0.5343, Figure 1D). Thus, we thought that soy peptides suppressed the IL-1β-induced production of MMP-3 and ADAMTS-4 by inhibiting their common pathway. IL-1β activates the signal transduction systems through the activation of multiple protein kinases such as p38 mitogen-activated protein kinase (MAPK), c-Jun N-terminal kinase (JNK), and Akt [15-18]. The activated MAPK, JNK, and Akt enhance production of various inflammatory cytokines and matrix degrading proteins, activating transcription factors such as AP-1 and NF-kB [19]. Akt, phosphorylated by activated phosphoinositol 3-kinase (PI3K), leads to activation of NF-kB [20]. NF-kB activates transcription of pro-inflammatory cytokines and matrix-degrading enzymes including MMP-3 and ADAMTS-4 [4,5]. Peptides derived from β-conglycinin of a representative soy protein have been reported to inactivate NF-kB by suppressing phosphorylation of Akt in liver and aorta of LPS-induced inflammatory model mice [11]. Thus, we speculate that soy peptides suppress the IL-1β-induced production of MMP-3 and ADAMTS-4, probably by inhibiting their PI3K-Akt-NF-kB signaling by suppressing the phosphorylation of Akt.
On the third possible mechanism, although receptors for soy peptides have not yet been identified, such receptors may exist and deliver signals. For example, M. Chimen et al. reported that PEPITEM, a peptide proteolytically derived from 14.3.3 zeta delta protein, used cadherin-15 as a receptor and lead to release of sphingosine-1-phosphate from endothelial cells [21]. Like the case of PEPITEM, receptors for soy peptides may exist, which should be investigated. Including these 3 possible mechanisms, the mechanisms for the action of soy peptides should be investigated in the near future.
We here found that soy peptides significantly suppressed the IL-1β-induced production of MMP-3 and ADAMTS-4 in human articular chondrocytes. In the majority of the tested chondrocytes samples, both the mRNA levels of MMP-3 and those of ADAMTS-4 were suppressed by the treatment with 100 µM soy peptides (Figure 1C). However, in a small part of the tested chondrocytes samples, neither the mRNA levels of MMP-3 nor those of ADAMTS-4 were suppressed by the soy peptides treatment (Figure 1C). Thus, the effect of soy peptides would be influenced by the individual genetic backgrounds. There might be subpopulations that show different sensitivity to soy peptides.
It is interesting that the suppressive effect of the soy peptides on the expression of MMP-3 and ADAMTS-4 tended to be higher in the patients with OA than in those with bone fracture (Figure 1D). OA chondrocytes might show higher sensitivity to soy peptides than normal chondrocytes.
Soy peptides used in this study are mainly composed of di- and tri-peptides [14]. However, specific peptides that suppressed the IL-1β-induced production of MMP-3 and ADAMTS-4 have not been identified yet. Recently, a tri-peptide of Val-Pro-Tyr, derived from glycinin of a major soy protein, has been reported to down-regulate the expression of pro-inflammatory cytokines in intestinal epithelial cells and macrophages [22]. The soy peptides used in this study are expected to contain glycinin and thus would contain Val-Pro-Tyr. A tri-peptide of Val-Pro-Tyr might be one of the soy peptides that suppressed the effects of IL-1β in this study. On this point, further investigation is required.
Our data showed that soy peptides suppressed the IL-1β-induced production of MMP-3 and ADAMTS-4 in human articular chondrocytes in vitro. Our data indicate that soy peptides may be useful to prevent or suppress cartilage degradation in OA.
Acknowledgements
We thank Ms. Sawada Y, Ms. Tamaki M, Ms. Mogi T, and Ms. Suzuki M for their technical assistance. This work was supported by FUJI OIL CO., LTD.
Conflict of interest statement
Arito M, Mitsui H, Kurokawa MS, Niki H, Yudoh K, and Kato T report grants and non-financial support from FUJI OIL Co. Ltd., during the conduct of the study. Kato T reports grants and non-financial support from Chugai Pharmaceutical Co. Ltd., outside the submitted work. Yudoh K reports non-financial support from Vitamin C60 BioResearch Corporation, outside the submitted work. Kamada T has nothing to disclose.
References
- Loeser RF, Goldring SR, Scanzello CR, Goldring MB (2012) Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum 64: 1697-1707. [Crossref]
- Goldring MB, Marcu KB (2009) Cartilage homeostasis in health and rheumatic diseases. Arthritis Res Ther 11: 224. [Crossref]
- Kaneyama K, Segami N, Nishimura M, Suzuki T, Sato J (2002) Importance of proinflammatory cytokines in synovial fluid from 121 joints with temporomandibular disorders. Br J Oral Maxillofac Surg 40: 418-423. [Crossref]
- Richardson DW, Dodge GR (2000) Effects of interleukin-1beta and tumor necrosis factor-alpha on expression of matrix-related genes by cultured equine articular chondrocytes. Am J Vet Res 61: 624-630. [Crossref]
- Bau B, Gebhard PM, Haag J, Knorr T, Bartnik E, et al. (2002) Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum 46: 2648-2657. [Crossref]
- Inoue N, Nagao K, Sakata K, Yamano N, Gunawardena PE, et al. (2011) Screening of soy protein-derived hypotriglyceridemic di-peptides in vitro and in vivo. Lipids Health Dis 10: 85. [Crossref]
- Maebuchi M, Kishi Y, Koikeda T, Fruya S (2013) Soy Peptide Dietary Supplementation Increases Serum Dopamine Level and Improves Cognitive Dysfunction in Subjects with Mild Cognitive Impairment. Jpn Pharmacol Ther 41: 67-73.
- Katayama S, Imai R, Sugiyama H, Nakamura S (2014) Oral administration of soy peptides suppresses cognitive decline by induction of neurotrophic factors in SAMP8 mice. J Agric Food Chem 62: 3563-3569. [Crossref]
- Matemu AO, Nakamura K, Kayahara H, Murasawa H, Katayama S, et al. (2011) Enhanced antiviral activity of soybean ß-conglycinin-derived peptides by acylation with saturated fatty acids. J Food Sci 76: M299-M304.[Crossref]
- Martinez-Villaluenga C, Dia VP, Berhow M, Bringe NA, Gonzalez de Mejia E (2009) Protein hydrolysates from beta-conglycinin enriched soybean genotypes inhibit lipid accumulation and inflammation in vitro. Mol Nutr Food Res 53: 1007-1018. [Crossref]
- Burris RL, Ng HP, Nagarajan S (2014) Soy protein inhibits inflammation-induced VCAM-1 and inflammatory cytokine induction by inhibiting the NF-κB and AKT signaling pathway in apolipoprotein E-deficient mice. Eur J Nutr 53: 135-148. [Crossref]
- Young D, Ibuki M, Nakamori T, Fan M, Mine Y (2012) Soy-derived di- and tripeptides alleviate colon and ileum inflammation in pigs with dextran sodium sulfate-induced colitis. J Nutr 142: 363-368. [Crossref]
- Kellgren JH, Lawrence JS (1957) Radiological assessment of osteo-arthrosis. Ann Rheum Dis 16: 494-502. [Crossref]
- Nakamori T (2010) Soy peptides as functional food materials. In: Bioactive proteins and peptides as functional foods and nutraceuticals. (Mine Y, Li-Chan ECY, Jiang B, eds.) Wiley-Blackwell Publishing, Hoboken, pp. 265-271
- Guy GR, Philp R, Tan YH (1995) Activation of protein kinases and the inactivation of protein phosphatase 2A in tumour necrosis factor and interleukin-1 signal-transduction pathways. Eur J Biochem 229: 503-511. [Crossref]
- Bankers-Fulbright JL, Kalli KR, McKean DJ (1996) Interleukin-1 signal transduction. Life Sci 59: 61-83. [Crossref]
- Li X, Tupper JC, Bannerman DD, Winn RK, Rhodes CJ, et al. (2003) Phosphoinositide 3 kinase mediates Toll-like receptor 4-induced activation of NF-kappa B in endothelial cells. Infect Immun 71: 4414-4420. [Crossref]
- Tsuzaki M, Guyton G, Garrett W, Archambault JM, Herzog W, et al. (2003) IL-1 beta induces COX2, MMP-, -3 and -13, ADAMTS-4, IL-1 beta and IL-6 in human tendon cells. J Orthop Res 21: 256-264. [Crossref]
- Williams DH, Jeffery LJ, Murray EJ (1992) Aurothioglucose inhibits induced NF-kB and AP-1 activity by acting as an IL-1 functional antagonist. Biochim Biophys Acta 1180: 9-14. [Crossref]
- Yap TA, Garrett MD, Walton MI, Raynaud F, de Bono JS, et al. (2008) Targeting the PI3K-AKT-mTOR pathway: progress, pitfalls, and promises. Curr Opin Pharmacol 8: 393-412. [Crossref]
- Chimen M, McGettrick HM, Apta B, Kuravi SJ, Yates CM, et al. (2015) Homeostatic regulation of T cell trafficking by a B cell-derived peptide is impaired in autoimmune and chronic inflammatory disease. Nat Med 21: 467-475. [Crossref]
- Kovacs-Nolan J, Zhang H, Ibuki M, Nakamori T, Yoshiura K, et al. (2012) The PepT1-transportable soy tripeptide VPY reduces intestinal inflammation. Biochim Biophys Acta 1820: 1753-1763. [Crossref]

