Abstract
Purpose: to evaluate the effects of age related macular degeneration (ARMD) on electrophysiological tests and to correlate between electroretinogram (ERG) and optical coherence tomography (OCT).
Methods: Fifty control subjects (100 eyes) and hundred patients (100 eyes) with ARMD were examined clinically. Electroretinogram (EOG), multifocal ERG, standard ERG, fluorescein angiography and optical coherence tomography were done.
Results: Electroretinogram responses were decreased in all types of ARMD especially in geographic atrophy. The amplitudes of scotopic ERG were slightly decreased without change in implicit times. The amplitudes of oscillatory potential (Ops) were significantly decreased and photopic responses were also decreased in geographic atrophy, choroidal neovascularization(CNVs)and pigment epithelium detachment. MF-ERG shows abnormalities not only in central ring but also in all rings (central, paracentral and peripheral). There was negative correlation between central macular thickness(CMT) and amplitude of MFERG of central ring and positive correlation between CMT and latency of MFERG of central ring in CNVs and PED
Conclusion: Electrophysiological responses indicated a general reduction of retinal function in all parts of the retina (not only in the macula but in every parts of the retina) in ARMD.
Introduction
Age related macular degeneration develop as people become older particularly in people above the age 65, However, it can develop in age of forties and fifties. Women have ARMD are common in women than men, possibly because women live longer than men. Smoking and ultraviolet sunlight increase the risk of ARMD. In elderly, ARMD is the leading cause of legal blindness in western nations [1,2].
There are two main types of ARMD: wet and dry ARMD. Dry form is marked by occurrence of a well-defined progressive lesion or atrophy in the macula. Wet form is characterized by occurrence of abnormal choroidal neovascularisation under the retina and macula causing bleeding and leaking of fluid. This leads to bulging and lifting of the macula and distoration of central vision [3,4].
The exact causes of ARMD are still unknown. Electrophysiological tests are used to describe the site of retinal dysfunction [5,6]. Full field ERG and EOG measures are summed responses from different cells of larger areas of the retina while MFERG is a map function with high resolution within the central 30 degrees. It can measure local cone and rod mediated functional impairment at early and late stages of ARMD [7].
EOG had the advantages over ERG in the electrodes did not touch the surface of the eye [8]. The aims of the study are to investigate the electrodiagnostic, tomographic and angiographic findings in ARMD and to correlate visual acuity, central macular thickness and MFERG.
Subjects and methods
This study was carried out on patients attending the Out-Patient Clinic of Mansoura Ophthalmic Center during the period May 2013 to February 2014.The study included 200 eyes of 150patients (100eyes of 100 patients with age related macular degeneration(ARMD),100 eyes of 50 control subjects. All patients were carried out in accordance with the tenets of the Declaration of Helsinki (1989) of the world medical association. The study was approved by Mansoura University Hospital trust ethics committee. Each patient signed a written consent statement before joining the study.
ARMD was diagnosed when the following criteria were fulfilled: age >55, visual acuity ≤ 0.5 and fundus examination shows picture of drusen, geographic atrophy (GA), choroidal neovascularization (CNVs), pigment epithelium detachment (PED). Patients who had ocular diseases such as myopia >3 diopter, diabetes that may influence the ERG, EOG &OCT were excluded from the study. All subjects underwent complete ophthalmologic examination including: visual acuity (BCVA; logarithium of the minimum angle of resolution (log MAR), automated autorefractormetry (using Topcon autorefractor), ocular tension tonometry using Goldman Tonometer, slit lamp examination. Indirect and direct posterior segment examination were done using 90 D lens and Goldmann 3-mirror lens. Flourescien angiography were taken using (Topcon Coporation ,2000,TRC,50IX,Japan).OCT ,EOG and ERG were performed.
Optical coherence tomography (OCT)
OCT was done by (Topcon, 3 dimensional OCT-1000 USA). OCT measurements were performed using fiber optically Michelson interferometer with short coherence length super-luminescent diode source. OCT was performed with 512×128 scan pattern. Central macular thickness (CMT) including a circular 1mm radius area around the fovea was measured automated.
Electrophysiological tests
Electrooculogram (EOG) and electroretinogram (Full field ERG and MF-ERG) were recorded using Roland Consult, Brandenburg and Germany system. During electrophysiological tests, monitoring of the fixation is allowed through fundus viewing. Electrophysiological tests were performed in accordance to International Society for Clinical Electrophysiology of Vision (ISCEV)
Pupils were fully dilated (>7 mm). After topical corneal anesthesia (Benoxinate hydrochloride 4%). Dawson, Trick and litzkow (DTL) electrode (positive electrode) was placed just contact with corneal limbus, ground electrode was installed on the forehead and negative electrode was applied near orbital rim temporary. Before placing electrodes, the skin was cleaned with cleaning rub cream. The electrodes should be installed under dim red light and after dark adaptation for 20 minutes. The recording was monocular and contra-lateral eye was occluded with light pressure to suppress blinking.
Full field ERG
The body position should be comfortable, the head is fixed before stimulator and the eyes fixate on red light point in the Ganzfeld globe, then test was started and recorded in 5 steps: scotopic rod response, scotopic combined response, oscillatory potential(Ops) then light adaptation for 10 minutes then photopic cone response and flicker response recording.
MF-ERG
Subjects were positioned 30cm from the stimulus monitor. The stimulus was presented on 32 X 22 cm monitor driven at a 75Hz frame rate and constitutes of an array of 61 hexagonal elements across a field (44º horizonitally × 40º vertically). Luminance of white hexagons were 185 to 200 cd/m2 and of dark hexagons were 1 to 2 cd/m2 resulting in local contrasts of 98% to 99%. Each hexagon was modulated between light and dark according to binary m-sequence. At any time, approximately 50% of the stimulus elements displayed were white and 50% were black. The patients fixated at a spot in the center of the stimulus during 8-minute recording sessions. Each session was continued to 30 second then followed by brief rest periods to improve fixation stability. Signals were amplified (gain, 106), band pass filtered (10-300 Hz). Two recordings were obtained from each eye. Recording segments contain two amplifier artifacts were discarded.
The results of two recordings were averaged to improve the signal to noise ratio. Amplitude was measured as the voltage difference between first trough and the first peak of scaled template wave. Implicit time was calculated to the first prominent response peak of the wave. The P component of the first order kernel of the MFERG was analyzed using software. These arrays were divided into 5-concentric rings centered on the fovea.
Electroculogram (EOG)
The test subject should be in normal indoor lighting for 60 minute before the test without exposure to any strong light. After dark adaptation for 15 minutes and pupil dilation, the electrodes were placed close to canthi of each eyes. Each eye needs two electrodes after cleaning skin with alcohol. The ground electrode was placed on forehead.
The patient put his chin on Ganzfeld Stimulator and move his eyes with fixation light (which consists of two red fixation lights 15 degrees left and right of a central light). The fixation lights should be as dim as practical in dark. After dark phase; the stable white light is on. 15 minutes are needed for light adaptation before light phase is started. The light luminance should be calibrated regularly. When the light adapting background is on the fixation lights should be bright.
Before recording, the subject was taught how to fixate on the two alternate fixation lights with stable pacing. Both during dark phase and light phase when lights change, eyes move in single sweep to next one every one second for ten times in one minute, the left time for rest.
In each saccade, there may overshoot (appears when eyes exceed the fixation lights and then go back stable position). The amplitude of saccades is measured automated. The light peak amplitudes (from light peak to baseline) the dark trough amplitudes (from dark peak to baseline) and Arden ratio (amplitude ratio of light peak to baseline).
Statistical analysis
EOG: The dark trough, light peak and Arden ratio values were detected from each recording.
Full field ERG: Amplitudes and implicit times of the rods and cones responses were detected. Also, the amplitude of Ops component and the mean peak to peak amplitude of flicker ERG were measured.
MFERG: Amplitude and implicit time of P wave over rings were determined.
OCT: central macular thickness was determined. Data from ARMD patients and normal controls were analysed SPSS (statistical package For social science). Qualitative data was presented as number. Chi square (x2) test of significance was used for comparison. Quantitative data was represented as mean ± standard deviation. Krauskal- Wallis, Munn Whitney and kolmogorov-smirnov tests were used for comparison. Spearman's correlation coefficient was used to calculate correlation between central macular thickness and electroretinogram values. Difference were considered statistically significant when P<0.05.
Results
Fifty normal subjects (100 eyes) and hundred patients (100 eyes) with ARMD were included in the study. The mean age was 55 ± 12.5 years in control group and 54 ± 14.9 years in ARMD.
Visual acuity
The mean best corrected visual acuity in ARMD was 0.49 ± 0.5 and in control group was 0.98 ± 0.22
Flourescien angiography
Drusen was found in 30 eyes, geographic atrophy(GA) in 30 eyes, PED in 10 eyes and choroidal neovascularisation in 30 eyes (15eyes were well defined CNVs and15eyes were occult CNVs)
Electrophysiology
EOG: The value of EOG dark trough was (in control 510 ± 30 µv versus in ARMD 227.5 ± 30 µv), light peak (450 ± 59 µv in ARMD versus 998 ± 50 µv in control) and Arden ratio (1.5 ± 0.4 µv in ARMD versus 2.5 ± 0.59 µv in control). The values were significantly lower in ARMD group than in control (Table 1, Figure 1).
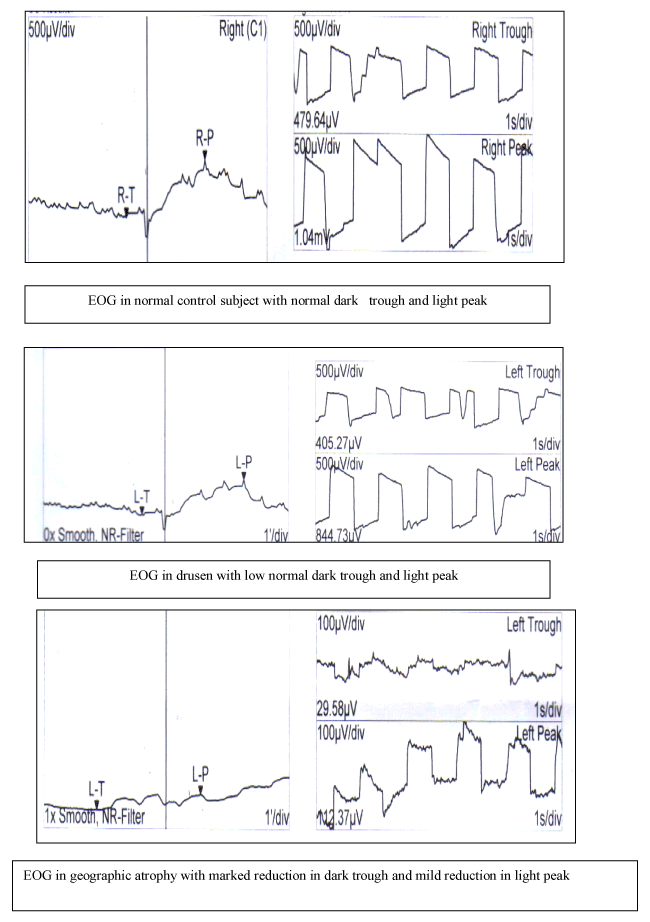
Figure 1. EOG among groups.
Table 1. Electro-oculogram changes among groups.
Groups |
Light Peak |
Dark trough |
Arden ratio |
Control |
998 ± 50µv |
510 ± 30 µv |
2.5 ± 0.59 |
ARMD |
|
|
|
Drusen |
750 ± 45 µv |
400 ± 40 µv |
2.4 ± 0.4 |
Geographic atrophy |
150 ± 60 µv |
50 ± 20 µv |
2.9 ± 0.9 |
CNVs |
405 ± 30 µv |
210 ± 42 µv |
1.5 ± 0.3 |
PED |
480 ± 30 µv |
450 ± 44 µv
|
0.9 ± 0.4 |
P |
0.001 |
0.005 |
0.008 |
Table 2. Full field ERG changes among groups.
ERG Parameters |
Control |
Drusen |
Geographic
atrophy |
CNVs |
PED |
Scotopic
b-wave
Amplitude (0.001) Implicit time (P=0.1) |
85 ± 13nv
60 ± 10 |
72 ± 10nv
65 ± 6.6 |
40 ± 5.5
75 ± 9.9 |
38.8 ± 7.6
92 ± 9.1 |
39 ± 8.5
90 ± 8.3 |
Combined
a-wave
Amplitude (P=0.22)
Implicit time(p=0.4)
b-wave
Amplitude (P=0.005)
Implicit time (P=0.31) |
110 ± 9.9
20 ± 4.9
230 ± 30nv
40 ± 8 |
100 ± 5.2
21 ± 4.6
200 ± 20
36 ± 6.3 |
90 ± 12.1
22 ± 3.8
120 ± 20.3
46 ± 12.1 |
88 ± 10.9
23 ± 3.3
120 ± 15.5
44 ± 5.6 |
85 ± 9.8
23 ± 4.1
122 ± 17.6
45 ± 6.1 |
Ops (P=0.007)
Implicit time |
23±2msec |
25 ± 3ms |
15±3.8 |
14±4.2 |
14.4±3.9 |
Amplitude |
27 ±3nv |
24 ±2nv |
30±4.6 |
32±5.4 |
33±5.2 |
Photopic
a-wave (.0009)
Amplitude
Implicit time
b-wave
Amplitude (P=0.001)
Implicit time (P=0.8) |
45 ± 5.8
15 ± 3.3
50 ± 9nv
30 ± 5.3 |
43 ± 5nv
16 ± 4.1
49 ± 5.4
33 ± 3.1 |
25 ± 6.8
30 ± 5.7
26 ± 7.7
35 ± 4.9 |
21.2 ± 5.8
31.4 ± 4.9
25.2 ± 8.9
39 ± 5.1 |
22 ± 6.1
32 ± 5.1
24 ± 7.7
38 ± 4.6 |
30Hz flicker
Implicit time |
50 ± 5msec |
51 ± 4.7ms |
60 ± 3.4 |
70 ± 5.3 |
69.8 ± 4.9 |
Amplitude |
55 ± 6nv |
50 ± 10nv |
40 ± 9.8 |
30 ± 8.9 |
29 ± 8.5 |
Table 3. MF-ERG amplitudes and latencies over rings.
Ring 5 |
Ring 4 |
Ring 3 |
Ring 2 |
Ring 1 |
Groups |
|
|
|
|
|
Amplitude |
30 ± 4.00 |
35 ± 5 |
44 ± 7 |
53 ± 6 |
65 ± 10 |
Control (P=0.002) |
|
|
|
|
|
ARMD (P=0.001) |
30 ± 5 .00 |
33 ± 5.00 |
42 ± 8.00 |
54 ± 6.00 |
64 ± 9.00 |
Drusen |
20 ± 3.00 |
22 ± 4.00 |
30 ± 2.00 |
31 ± 3.00 |
33 ± 5.00 |
Geographic atrophy |
16 ± 5.00 |
17 ± 3.00 |
18 ± 4.00 |
19 ± 5.00 |
30 ± 6.00 |
PED |
10 ± 6.00 |
11 ± 5.00 |
16 ± 6.0 |
15 ± 7.0 |
25 ± 5.0 |
CNVs |
40 ± 0.8 |
40 ± 0.9 |
37 ± 0.5 |
36 ± 1 |
35 ± 0.9 |
Latencies
Control (P=0.001) |
|
|
|
|
|
ARMD(P=0.000) |
40 ± 1 |
39 ± 0.8 |
37 ±0.9 |
35 ± 1 |
36 ± 0.7 |
Drusen |
55 ± 1.0 |
55 ± 1.2 |
52 ± 1.0 |
49 ± 1 |
50 ± 3 |
Geographic atrophy |
54 ± 1.0 |
54 ± 1 |
54 ± 2.1 |
53 ± 2 |
52± 4 |
PED |
56 ± 2 |
56 ± 3 |
53 ±1.5 |
52 ± 2 |
55 ± 3 |
CNVs |
Table 4. Central macular thickness (CMT) among groups.
CMT |
Groups |
170 ± 9 |
Control (P=0.001) |
|
ARMD (P=0.000) |
180 ± 14 |
Drusen |
250 ± 50 |
Geographic atrophy |
301 ± 100 |
PED |
335 ± 90 |
CNVs |
Table 5. Correlation between CMT and visual acuity and MFERG.
Ring 5 |
Ring 4 |
Ring 3 |
Ring 2 |
Ring 1 |
Groups |
0.00 |
0.009 |
0.001 |
0.001 |
0.000 |
Amplitude
CMT P |
-0.4 |
-0.4 |
-0.5 |
-0.5 |
-0.55 |
R |
0.06 |
0.05 |
0.005 |
0.002 |
0.001 |
Implicit time
P |
0.4 |
0.4 |
0.51 |
0.5 |
0.5 |
R |
0.003 |
0.005 |
0.002 |
0.001 |
0.000 |
Amplitude
V.A P |
-0.42 |
-0.42 |
0.52 |
0.55 |
0.65 |
R |
0.06 |
0.05 |
0.08 |
0.02 |
0.01 |
Implicit time
P |
-0.34 |
-0.44 |
-0.451 |
-0.445 |
-0.45 |
R |
In drusen, light peak values were normal (Figure 2) but the values in eyes with geographic atrophy were subnormal. Thus, in drusen light rise was greater than in geographic atrophy. The lowest values of dark trough were in geographic atrophy while highest values were found in PED. In drusen, the elevation of light rise in eyes was significantly greater than in other groups (Table 1).
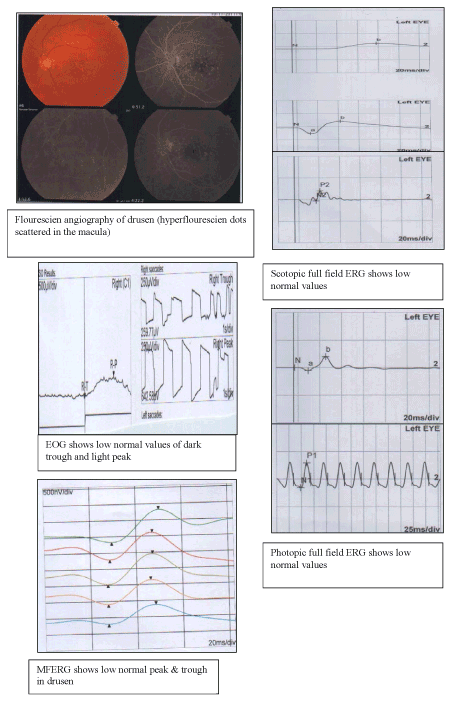
Figure 2. A case of drusen with normal ERG & EO.
Full field ERG: In drusen, the amplitudes of all parameters of full field were within lower normal (Figure 2) but implicit time of scotopic b-wave was mildly reduced. A-wave of combined flashed response in ARMD did not different significant. However, amplitudes of scotopic b-wave and b-wave of combined responses were significantly reduced in GA, CNVs, PED. Whereas, implicit times of b-waves were within normal. Also, the amplitudes of oscillatory potentials were decreased in ARMD (GA, CNVs, PED). The amplitudes of photopic a-and b-waves were significantly lower in ARMD (GA, CNVs, PED), while implicit time of a-wave was significantly prolonged and the implicit time of b-wave was normal. The amplitude of the flicker ERG was also reduced (Table 2).
MF-ERG: The MFERG recordings show marked reduction in the site of GA, CNVs, PED (as revealed by fundus photography and flourescein angiography) and mild reduction in the surrounding rings. The trace array and response density maps were subnormal especially in the site of lesion and within the lower normal level in drusen (Table 3, Figure 3,4).
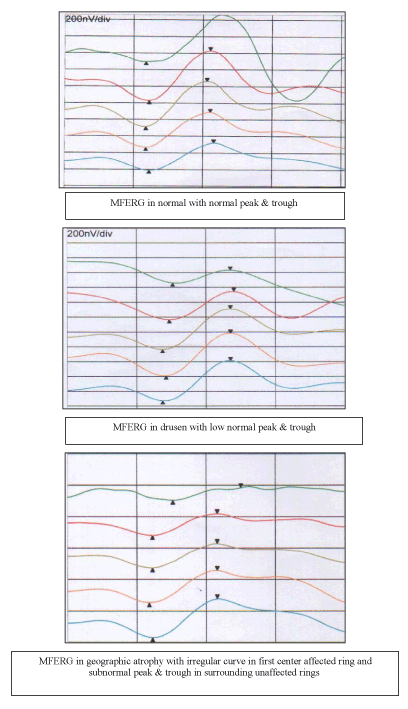
Figure 3. MFERG in ARMD.

Figure 4. Fluorescein angiography, OCT EOG and MFERG in CNV.
OCT: The mean central macular thickness (CMT) in drusen was near normal values (CMT=180 ± 14) while CMT were 335 ± 90 in CNVs and 301 ± 100 in PED. There was negative correlation between CMT and amplitude of MFERG of central ring and positive correlation between CMT and latency of MFERG of central ring in CNVs and PED. The increase in CMT is accompanied with increase in implicit times and decrease in amplitude of MFERG. There was correlation between amplitudes of MFERG and sizes of GA, CNVs, PED (P=0.002, R=0.55). There was no significant correlation between CMT and MFERG in drusen (p=0.5.). There was significant correlation between the size of the lesion and amplitudes over the affected area (Table 4,5).
Discussion
Electrophysiological tests show the complicated interaction between choroids, RPE, and receptor layer on one side and on the postsynaptic layers on the other side [9]. Full field ERG is a general response from the retina while MF-ERG gives a series of localized waves from single recording and allows the assessment of spatial information across the macula [10].
EOG provides additional information on the function of retinal pigment epithelium. The disadvantage is the high inter- and intra-individual variability of the results [11]. In this study, there were normal EOG (normal light rise values, dark trough values and Arden ratio), normal full field ERG and normal MFERG in drusen, but the normal values in drusen were within the lower limit. These results of ERG suggest that retinal pigment epithelial electrophysiologic function is well maintained in drusen despite the wide spread physical abnormalities of the retina pigment epithelium.
Also, in this study, in drusen implicit times were shorter than other sub-groups. The cause is that the features of photoreceptors in drusen were slightly different than other types of ARMD. In drusen, photo-transduction may yield a faster ion exchange initiating a faster synaptic transmission to bipolar cells. Similarly, Gupta and Marmor found that there was no significant difference between the normal subjects and patients with drusen [12]. Also, Fishman et al. [13] and Rover et al. [14] said that EOG was close to normal. Normal EOG was presented in all cases of drusen, that means that the disease did not affected the retinal pigment epithelium [13,14]. In contrast, Walter et al. [15], found that dark trough was reduced in eyes with drusen.
In this study, in geographic atrophy, there were reduction in full-field ERG and MFERG parameters. Lowest dark trough was seen in these cases. Similarly, Walter et al. [15,16], and Friedman said that there decrease in the values of full-field ERG and EOG in cases of geographic atrophy. In the opposite, Marcus et al. [17] found reduction of light rise in only one of 12 patients with ARMD. In this study, there were reduction in EOG ,full field ERG and MFERG values. The reduction in MFERG was not only in the ring of the site of lesion but also in the surrounding rings in cases of PED. The cause of global retinal dysfunction in ARMD may be vascular [18-20]. The same as walter et al found reduction in EOG and full field ERG in PED [15]. In this study, in cases of CNVs, there were reduction in all values of EOG, full field ERG and MFERG. In MFERG, there were marked reduction in amplitude and prolongation in implicit times over affected ring and mild reduction in the surrounding rings.
Also, Schouten et al. [21], Maturi et al. [22], Oh et al. [23] and Park et al. [24] observed reduction in response densities to less than normal values and prolongation of implicit time. The number and function of photoreceptor cell is responsible for p amplitude reduction [25]. While, bipolar cell response of the outer retina may be responsible for P implicit time delay [26]. Hood thought that the implicit time is affected more than the amplitude to damage of photoreceptors and the outer plexiform layer [27].
Maturi et al. [28] said that the origin of MFERG is the cells of the outer retina rather than cells from the inner retina. Similarly, Li et al. [25] observed reduction in retinal function in early ARMD, pre-age macular degeneration and in fellow eye with normal fundus appearance. This indicated general functional affection of both eyes and areas beyond the sites of visible lesions in the affected eye. While, Jurklies et al. [29] found a reduction of MFERG response densities in the site of CNVs. The amplitude in the ring 3-5 were within low normal range (less than 2 standard deviations below the mean value).
In this study, as regards ERG, there were significant differences between ARMD patients (except drusen) and controls. Both scotopic and photopic responses were reduced and prolonged indicating that in ARMD not only cones were impaired but also the rod system. The oscillatory potentials were severely affected. In this study, there were highly significant positive correlation between MFERG amplitude over CNVs, PED and GA and visual acuity, while there was statistically insignificant correlation between the visual acuity of the patients and the size of CNVs, PED and GA indicating that the size of the lesion does not reflect its effect on retinal function.
Similarly, Jurklies et al. [29] and Park et al. [24] found strong correlation between MFERG response and visual acuity and weak correlation between MFERG response and lesion size. In summary, in ARMD not only local responses were impaired, but also general responses were affected indicating that in these patient’s larger retinal area was affected than one may suspect from the fundus appearance. MFERG allows topographic mapping and objective assessment retinal function within as well as outside the fovea. It allows estimation the extent of retinal dysfunction within central 30º of retina. MFERG is used to obtain local electrophysiological response of central retina while full field ERG gives information about general retinal function.
References
- Gibson JM, Lavery JR, Rosenthal AR (1986) Blindness and partial sight in an elderly population. Br J Ophthalmol 70: 700-705. [Crossref]
- Klein R, Klein BEK, Wang Q, Moss SE (1992) Prevelence of age related maculopathy: the Beaver Dam Eye Study. Ophthalmol 99: 933-943. [Crossref]
2021 Copyright OAT. All rights reserv
- Gragoudas ES, Adamis AP, Cunningham ET Jr, Feinsod M, Guyer DR; VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group (2004) Pegaptanib for neovascular age-related macular degeneration. N Engl J Med 351: 2805-2816. [Crossref]
- Lamoureux EL, Mitchell P, Rees G, Cheung G, Yeo I, et al. (2011) Impact of early and late age-related macular degeneration on vision-specific functioning. Br J Ophthalmol 95: 666-670. [Crossref]
- Seiple WH, Siegel IM, Carr RE, Mayron C (1986) Evaluating macular function using the focal ERG. Invest Ophthalmol Vis Sci 27: 1123-1130. [Crossref]
- Hood DC, Holopigian K, Greenstein V, Seiple W, Li J, et al. (1998) Assessment of local retinal function in patients with retinitis pigmentosa using the multi-focal ERG technique. Vision Res 38: 163-179. [Crossref]
- Feigl B, Lovie-Kitchin J, Brown B (2005) Objective functional assessment of age-related maculopathy: a special application for the multifocal electroretinogram. Clin Exp Optom 88: 304-312. [Crossref]
- Sutter EE, Tran D (1992) The field topography of ERG components in man--I. The photopic luminance response. Vision Res 32: 433-446. [Crossref]
- Kondo M, Miyake Y, Horiguchi M, Suzuki S, Tanikawa A (1997) Recording multifocal electroretinograms with fundus monitoring. Invest Ophthalmol Vis Sci 38: 1049-1052. [Crossref]
- Rüther K, Breidenbach K, Schwartz R, Hassenstein A, Richard G (2003) [Testing central retinal function with multifocal electroretinography before and after photodynamic therapy]. Ophthalmologe 100: 459-464. [Crossref]
- Alanko HI (1984) Clinical electro-oculography. Acta Ophthalmol Suppl 161: 139-148. [Crossref]
- Gupta LY, Marmar MF (1994) Sequential recording of photic and non photic electrooculogram responses in patients with extensive extramacular drusen. Doc Ophthalmol 88: 49-55.
- Fishman GA, Carrasco C, Fishman M (1976) The electro-oculogram in diffuse (familial) drusen. Arch Ophthalmol 94: 231-233. [Crossref]
- Röver J, Bach M (1987) C-wave versus electrooculogram in diseases of the retinal pigment epithelium. Doc Ophthalmol 65: 385-391. [Crossref]
- Walter P, Widder RA, Lüke C, Königsfeld P, Brunner R (1999) Electrophysiological abnormalities in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 237: 962-968. [Crossref]
- Friedman E (1997) A hemodynamic model of the pathogenesis of age-related macular degeneration. Am J Ophthalmol 124: 677-682. [Crossref]
- Marcus M, Merin S, Wolf M, Feinsod M (1983) Electrophysiologic tests in assessment of senile macular degeneration. Ann Ophthalmol 15: 235-238. [Crossref]
- Remulla JFC, Gaudio AR, Miller S, Sandberg MA (1995) Foveal electroretinograms and choroidal perfusion characteristics in fellow eyes of patients with unilateral neovascular age-related macular degeneration. Br J Ophthalmol 75: 558-561. [Crossref]
- Grunwald JE, Hariprasad SM, DuPont J, Maguire MG, Fine SL, et al. (1998) Foveolar choroidal blood flow in age-related macular degeneration. Invest Ophthalmol Vis Sci 39: 385-390. [Crossref]
- Brown B, Tobin C, Roche N, Wolanowski A (1986) Cone adaptation in age-related maculopathy. Am J Optom Physiol Opt 63: 450-454. [Crossref]
- Schouten JS, La Heij EC, Webers CA, Lundqvist IJ, Hendrikse F (2009) A systematic review on the effect of bevacizumab in exudative age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 247: 1-11. [Crossref]
- Maturi RK, Bleau LA, Wilson DL (2006) Electrophysiologic findings after intravitreal bevacizumab (Avastin) treatment. Retina 26: 270-274. [Crossref]
- Oh SB, Cho WB, Moon BJ (2009) Effects and prognostic factors of intravitreal bevacizumab on choroidal neovascularization from age –related macular degeneration. J Korean Ophthalmol Soc 50: 202-210.
- Park JY, Kim SH, Park TK, Ohn YH (2011) Multifocal electroretinogram findings after intravitreal bevacizumab injection in choroidal neovascularization of age-related macular degeneration. J Korean Ophthalmol 25: 161-165. [Crossref]
- LiJ, Tso Mo, Lam TT (2001) Reduced amplitude and delayed latency in foveal response of multifocal electroretinogram in early age related macular degeneration. Br J Ophthalmol 85: 287-290. [Crossref]
- Hood DC, Frishman LJ, Saszik S, Viswanathan S (2002) Retinal origins of the primate multifocal ERG: implications for the human response. Invest Ophthalmol Vis Sci 43: 1673-1685. [Crossref]
- Hood DC, Odel JG, Chen CS, Winn BJ (2003) The multifocal electroretinogram. J Neuroophthalmol 23: 225-235. [Crossref]
- Maturi RK, Yu M (2003) Multifocal electroretinogram and its clinical application. In: Ciulla Regillo CD, Harris A, editors. Retina and optic nerve imaging. Philadelphia: Lippin Cott Williams & Wilkins 213-230.
- Jurklies B, Weismann M, Hüsing J, Sutter EE, Bornfeld N (2002) Monitoring retinal function in neovascular maculopathy using multifocal electroretinography early and long term correlation with clinical findings. Graefe’s Arch Clin Exp Ophthalmol 240: 244-264. [Crossref]




