Fibroin is the main polypeptidic component of the silk fibre generated by the larvae of the domesticated silk moth (Bombyx mori), and it has been extensively studied as a biomaterial with applications in tissue engineering and regenerative medicine. Due to their inherent practical advantages, the hydrogels constitute the preferred format for the B. mori silk fibroin (BMSF) biomaterials, which can conveniently be obtained by crosslinking processes. While the physically or chemically crosslinked BMSF hydrogels have been frequently described, the self-crosslinking of BMSF solutions induced by the catalytic effect of the enzyme horseradish peroxidase (HRP) has been barely reported. Following a previous preliminary study, where we demonstrated the advantages of using this enzyme for crosslinking BMSF, in the present work we investigated factors (amount of enzyme, initial fibroin concentration) that may affect the gelation. The measurement of dynamic moduli resulting from application of an oscillatory shear stress in a rheometer was our method to estimate the gelation time and to investigate the influence of certain factors on the process of crosslinking. It was found that both a higher initial concentration of the BMSF solution and a higher amount of the catalyst HRP induced a significant reduction of the gelation time.
silk fibroin; enzymatic reaction; horseradish peroxidase; crosslinking; gel point
Bombyx mori silk fibroin (henceforth BMSF) has become one of the naturally-derived biomaterials with potentially major applications in tissue engineering and regenerative medicine [1-12]. We have recently investigated [13] the enzymatic crosslinking of BMSF in an aqueous medium with the aim of providing a convenient method for generating BMSF hydrogels. While the format of hydrogel is preferred in the applications of BMSF biomaterials, the process of gelation by sol–gel transition [14-16], related to changes in the conformational structures of the polypeptidic chains of BMSF, is slow and can take days to weeks. Other alternatives include [13] treatment with solvents or surfactants, techniques such as lyophilization or vortexing, or chemical crosslinking. Most of these procedures are indeed capable of shortening the time needed for attaining the gel point, but some are associated with potential chemical contamination due to traces of the agents employed in certain procedures. This is obviously detrimental to the biomedical applications of BMSF hydrogels.
The enzymatically-induced crosslinking of BMSF using as a catalyst the enzyme horseradish peroxidase (EC1.11.1.7), henceforth HRP, has been only recently reported as an alternative method to generate BMSF hydrogels [13, 17-19]. The HRP-catalyzed crosslinking of BMSF can offer distinctive advantages, including significantly shorter gelation times and enhanced biomaterial properties of the resulting hydrogels (elasticity, cytocompatibility, and biodegradability), that we have previously confirmed [13]. As some discrepancies could be noticed between the results of these studies [13, 17, 19], we therefore have endeavoured here to further investigate the subject in the hope of elucidating additional characteristics of the process.
In the present study, the time needed to attain the gel point (i.e. the gelation time) in the system HRP/hydrogen peroxide (H2O2)/BMSF was determined using a rheological method, by measuring the dynamic moduli in small-amplitude oscillatory shear stress experiments, where the evolution of the complex dynamic shear modulus (G*) was monitored by recording in a rheometer its real part (G’, the storage modulus) and its imaginary part (G”, the loss modulus) as functions of time (t) over the duration of crosslinking reaction. Tung and Dynes [20] were the first to propose that the gel point occurs at the crossover of G’ (t) and G” (t) plots recorded in a rheometer. Thanks to seminal theoretical and experimental work by Winter’s group [21-24], the rheological behaviour of a gel at the critical point of attaining instant gelation (i.e. the gel point) became better understood, and a confirmation of the validity of the G’-G” crossover as a quantitative estimation of the gelation time was confirmed in stoichiometrically balanced polymer networks, where the stress relaxation follows a power law as a function of t–1/2 [24-26]. In inbalanced systems, usually heterogeneous, such as those occurring in the phase-separation polymerization processes, this criterion is unlikely to remain valid, however an extrapolation at the onset of the G’(t) plot could be employed as an alternative reasonable estimation of the gel point in such a system [27]. In the system of our study, the time-dependent plots of the moduli G’ and G” and the G’-G” crossover were well defined. The crossover G’-G” was accordingly used to estimate the gel point occurrence in the reaction mixture HRP/H2O2/BMSF and to examine the effect of changing the amount of enzyme and the concentration of BMSF.
Materials
B. mori silk cocoons were supplied by Tajima Shoji C. Ltd. (Yokohama, Japan), all cut in half and with the pupae removed. Sodium carbonate, lithium bromide, and HRP Type VI (as lyophilized powder, Lot#SLBQ7205V) were supplied by Sigma-Aldrich (St Louis, MO, USA). Hydrogen peroxide (30%) was supplied by Ajax Finechem (Australia). The Minisart®-GF pre-filters (0.7 μm) and Minisart® filters (0.2 μm) were supplied by Sartorius Stedim Biotech (Göttingen, Germany). The dialysis cassettes Slide-A-Lyzer® (with MWCO 3.5 kDa) were supplied by Thermo Scientific (Rockford, IL, USA). Water of high purity (Milli-Q or equivalent grade) was used in all experiments where needed. All concentrations and compositions in this report are expressed in percentage by weight (w/w), unless otherwise specified.
HRP (specific activity 325 U for 1 mg solid) was dissolved in water to make a stock solution with a concentration of 150 U/mL. A solution of 0.3% hydrogen peroxide (H2O2) was obtained by diluting the original 30% solution. All solutions were stored at 4°C.
Preparation of BMSF solution
The solution of regenerated BMSF was prepared following a protocol established previously [28]. Briefly, the silk cocoons were degummed by boiling in a 0.02-M aqueous solution of sodium carbonate. The degummed fibres were dissolved in an aqueous solution of lithium bromide (9.3 M) at 60°C, and then dialyzed for 3 days. The resulting solution was filtered through successive Minisart® filters. Gravimetric analysis showed that, with minor variations between batches, the final solution had a concentration of 3% fibroin in water. The lower concentrations (1% and 2%) were obtained by appropriate dilution. All solutions were stored at 4°C.
Rheology of the crosslinking reaction
The crosslinking reactions were carried out directly in a Modular Compact Rheometer (MCR 320, Anton Paar, UK). The liquid samples, consisting of appropriate volumes of HRP (150 U/mL) and H2O2 (0.3%) solutions, added respectively to 0.5-mL portions of BMSF solutions (concentrations of 1, 2, or 3%), were transferred onto the rheometer’s lower glass plate after a rapid mixing (t=0), and the measurements commenced within 30 s. The formulations of the crosslinking mixtures are shown in Table 1. The moduli G’ and G” were recorded as a function of time, for an angular speed of 10 rad/s, at the constantly maintained temperature of 37°C (Table 1).
Table 1. Composition of the crosslinking mixtures showing the various amounts of HRP solution (150 U/mL) added to 0.5 mL of BMSF solutions of different concentrations, for a constant amount of 8 µL H2O2 (0.3% concentration).
|
Sample #
|
Concentration of BMSF solution
|
Volume of HRP added
|
Concentration of HRP in the reaction mixture
|
|
|
(%)
|
(µL)
|
(U/mL)
|
(µg/mL)
|
|
1
|
1
|
4
|
1.17
|
3.61
|
|
2
|
|
8
|
2.33
|
7.16
|
|
3
|
|
12
|
3.46
|
10.65
|
|
4
|
|
16
|
4.58
|
14.09
|
|
5
|
2
|
4
|
1.17
|
3.61
|
|
6
|
|
8
|
2.33
|
7.16
|
|
7
|
|
12
|
3.46
|
10.65
|
|
8
|
|
16
|
4.58
|
14.09
|
|
9
|
3
|
4
|
1.17
|
3.61
|
|
10
|
|
8
|
2.33
|
7.16
|
|
11
|
|
12
|
3.46
|
10.65
|
|
12
|
|
16
|
4.58
|
14.09
|
Figure 1 shows representative G’ (t) and G” (t) plots recorded during the crosslinking reaction in the rheometer. As their crossover was consistently well defined, we considered to use its projection on the time axis as an estimation of the gel point, although this issue requires some comments.
Based on a rather vast literature, the gel point of a crosslinked polymer system can be defined as the instant when the average molecular mass diverges to infinity in a sample of infinite size, or when the largest molecular cluster extends across a sample of finite size. A critical phenomenon, the gel–sol phase transition, takes place at the gel point. No independent state of matter exists at the gel point: the crosslinking polymer system is either still before the gel point (as a “sol”, i.e. a viscoelastic fluid) or already beyond it (as a “gel”, i.e. a viscoelastic solid). Historically, the determination of gel point has been the subject of much debate. Despite the restrictions to the G’-G” crossover criterion, as proposed by Winter [24], this criterion has been used in biopolymer [29, 30] or synthetic polymer [31] gelling systems where a stoichiometric balance was not clearly defined.
The HRP-catalyzed crosslinking of BMSF in an aqueous medium leads to chemical gels [13]. To define a balanced stoichiometric relation between the components of our system is a complicated task, therefore we cannot qualify our system either ideal or non-ideal for the application of G’-G” crossover criterion. However, whether or not the G’-G” crossover points in our study coincide with the real gel points of the crosslinking reaction is not a crucial issue for the simple reason that this is a comparative study.
Our results showed (Figure 1) that by increasing the concentration of the fibroin in the reaction mixture, for a given amount of HRP and a fixed amount of H2O2, the gelation time was significantly reduced. For instance, the gel point in a solution of 1% BMSF appeared two times later than that in a solution of 3% BMSF, for the same concentration of 1.17 U/mL (or 3.61 μg/mL) HRP, and the trend is maintained regardless of the amount of HRP. Figure 2 displays the effect of the amount of HRP added to the crosslinking solution. A dramatic reduction of the gelation time is evident, leading eventually to the lowest value achieved in our experiments of 17.5 min in the system consisting of 3% BMSF, 4.58 U/mL HRP and 8 μL H2O2 (0.3%).
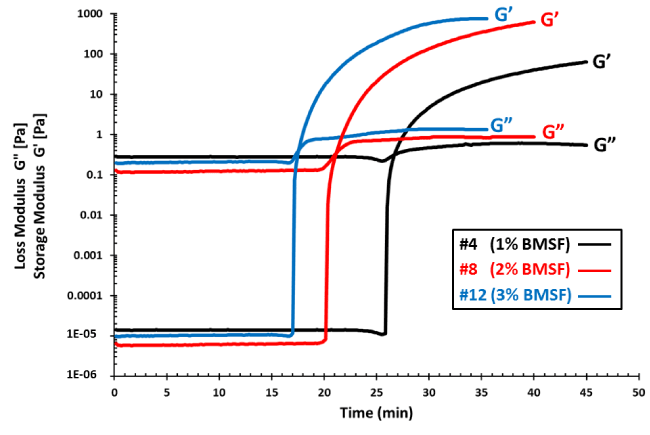
Figure 1. Time-dependent plots of the storage (G’) and loss (G”) moduli recorded during the HRP/H2O2-induced crosslinking of BMSF in an aqueous medium. The samples recorded here include #4, 8 and 12 (see the formulations in Table 1).
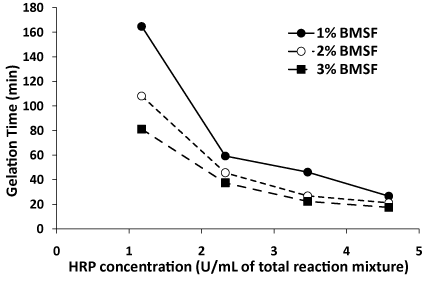
Figure 2. Gelation time of the crosslinking process as a function of the concentration of HRP in the reaction mixture for different concentrations of BMSF. Lines are guide to the reader’s eye only.
The crosslinking of silk fibroin catalyzed by the enzyme horseradish peroxidase is a clean and fast process for generating hydrogels of practical importance as biomaterials. The gelation time is much faster than in other procedures, and can be adjusted by changing the amounts of enzyme and/or the initial concentration of fibroin.
This work was supported by the Queensland Eye Institute Foundation (Brisbane, Australia) and by the project grant #1080302 from the National Health and Medical Research Council of Australia. The authors thank Emily Grundgeiger and Elizabeth Graham from the Central Analytical Research Facility at the Queensland University of Technology for assistance with rheological measurements, and to Dr. Tim Dargaville for valuable advice. The authors declare no conflict of interest related to this report.
- 1. Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL. 2003. Silk-based biomaterials. Biomaterials. 24: 401-416. [Crossref]
- 2. Vepari C, Kaplan DL (2007) Silk as a biomaterial. Prog Polym Sci 32: 991-1007. [Crossref]
- Hakimi O, Knight DP, Vollrath F, Vadgama P. 2007. Spider and mulberry silkworm silks as compatible biomaterials. Composites B. 38; 324-337.
- Murphy AR, Kaplan DL (2009) Biomedical applications of chemically-modified silk fibroin. J Mater Chem 19; 6443-6450. [Crossref]
- Harkin DG, George KA, Madden PW, Schwab IR, Hutmacher DW, Chirila TV. 2011. Silk fibroin in ocular tissue reconstruction. Biomaterials. 32: 2445-2458. [Crossref]
- Pritchard EM, Kaplan DL (2011) Silk fibroin biomaterials for controlled release drug delivery. Expert Opin Drug Deliv 8: 797-811. [Crossref]
- Wenk E, Merkle HP, Meinel L (2011) Silk fibroin as a vehicle for drug delivery applications. J Control Release 150: 128-141. [Crossref]
- Kundu B, Rajkhowa R, Kundu SC, W2021 Copyright OAT. All rights reservs for tissue regenerations. Adv Drug Deliv Rev 65: 457-470. [Crossref]
- Gil ES, Panilaitis B, Bellas E, Kaplan DL (2013) Functionalized silk biomaterials for wound healing. Adv Healthc Mater 2: 206-217. [Crossref]
- Kundu B, Kurland NE, Bano S, Patra C, Engel FB, Yadavalli VK, Kundu SC. 2014. Silk proteins for biomedical applications: bioengineering perspectives. Prog Polym Sci. 39:251-267.
- Koh LD, Cheng Y, Teng CP, Khin YW, Loh XJ, Tee SY, Low M, Ye E, Yu HD, Zhang YW, Han MY. 2015. Structures, mechanical properties and applications of silk fibroin materials. Prog Polym Sci. 46:86-110.
- Kapoor S, Kundu SC2 (2016) Silk protein-based hydrogels: Promising advanced materials for biomedical applications. Acta Biomater 31: 17-32. [Crossref]
- Chirila TV, Suzuki S, Papolla C. Forthcoming 2017. A comparative investigation of Bombyx mori silk fibroin hydrogels generated by chemical and enzymatic cross-linking. Biotechnol Appl Biochem.
- Jin HJ, Kaplan DL (2003). Mechanism of silk processing in insects and spiders. Nature 424: 1057-1061. [Crossref]
- Kim UJ, Park J, Li C, Jin HJ, Valluzzi R, et al. (2004) Structure and properties of silk hydrogels. Biomacromolecules 5: 786-792. [Crossref]
- Matsumoto A, Chen J, Collette AL, Kim UJ, Altman GH, et al. (2006) Mechanisms of silk fibroin sol-gel transitions. J Phys Chem B 110: 21630-21638. [Crossref]
- Yan LP, Oliveira AL, Oliveira JM, Pereira DR, Correia C, et al. inventors. A4TEC &Universidadedo Minho, assignees. 2013. Hidrogéis derivados da fibroína da seda: Métodos e respectivas aplicações. Portugal patent. PT 106041A.
- Yan LP, Correia C, Pereira DR, Sousa RA, Oliveira JM, et al. 2013. Injectable and dual-stimuli-responsive silk fibroin hydrogels for tissue engineering and regenerative medicine applications. J Tissue Eng Regen Med. 7(1Supp): 14S (abstract TS17).
- Partlow BP, Hanna CW, Rnjak-Kovacina J, Moreau JE, Applegate MB, et al. 2014. Highly tunable elastomeric silk biomaterials. Adv Funct Mater. 24:4615-4624. [Crossref]
- Tung CYM, Dynes PJ. 1982. Relationship between viscoelastic properties and gelation in thermosetting systems. J Appl Polym Sci. 27:569-574.
- Chambon F, Winter HH. 1985. Stopping of crosslinking reaction in a PDMS polymer at the gel point. Polym Bull. 13:499-503.
- Winter HH, Chambon F. 1986. Analysis of linear viscoelasticity of a crosslinking polymer at the gel point. J Rheol. 30:367-382.
- Chambon F, Winter HH. 1987. Linear viscoelasticity at the gel point of a crosslinking PDMS with inbalanced stoichiometry. J Rheol. 31:683-697.
- Winter HH. 1987. Can the gel point of a cross-linking polymer be detected by the G’-G” crossover? Polym Eng Sci. 27:1698-1702.
- Muller R, Gérard R, Dugand P, Rempp P, Gnanou Y. 1991. Rheological characterization of the gel point: A new interpretation. Macromolecules. 24:1321-1326.
- Mortimer S, Ryan AJ, Stanford JL. 2001. Rheological behaviour and gel-point determination for a model Lewis acid-initiated chain growth epoxy resin. Macromolecules. 34:2973-2980.
- Chirila TV, Higgins B, Dalton PD. 1998. The effect of synthesis conditions on the properties of poly(2-hydroxyethyl methacrylate) sponges. Cellular Polym. 17:141-162.
- Chirila TV, Barnard Z, Zainuddin, Harkin DG, Schwab IR, Hirst LW. 2008. Bombyx mori silk fibroin membranes as potential substrata for epithelial constructs used in the management of ocular surface disorders. Tissue Eng A. 14: 1203-1211. [Crossref]
- Djabourov M, Leblond J, Papon P. 1988. Gelation of aqueous gelatin solutions. II. Rheology of sol-gel transition. J Phys (France). 49:333-343.
- Rodd AB, Cooper-White J, Dunstan DE, Boger DV. 2001. Gel point studies for chemically modified biopolymer networks using small amplitude oscillatory rheometry. Polymer. 42:185-198.
- Higham AK, Garber LA, Latshaw DC, Hall CK, Pojman JA. 2014. Gelation and cross-linking in multifunctional thiol and multifunctional methacrylate systems involving an in situ comonomer catalyst. Macromolecules. 47:821-829.


