Abstract
The objectives were to evaluate the prevalence of Hidden Cervical Cancer (HCC), to analyze diagnostic and histopathological aspects, and clinical evolution of 677 patientswith cervical cancer,attended from January 1995 through December 2013. The design was retrospective and observational. The diagnostic methods used were cytology, colposcopy and histopathological study of surgical specimens. The HCC was found in 68/677 patients (10,04%): 55,9% of which consulted for a medical checkup, 33,8% for metrorragia, 19,1% for sinusorragia, 2,9% for bleeding and 1,5% for inguinal node; whereas 62% of the patients with exocervical or exo-endocervical cancer (EEC) consulted for bleeding, 23% for sinusorragia and vaginal discharge with odor, and 16% for a medical checkup (p< 0.0003). The cytology’s sensitivity for HCC was 47,1% (CI 95%, 35.2-59), 36,8% were false negatives; whereas for EEC it was 69,1% (CI 95%, 65.4-72.8) and 12,3% respectively (p< 0.001). The colposcopy’s sensitivity for HCC was 17,65% (CI 95%, 8.6-26.7); whereas for EEC it was 85,55% (CI 95%, 82.8-88.4; p< 0.0001). 30,9% of the cases of HCC were diagnosed by biopsies and 41,2% by surgical procedures; whereas 89,3% of the cases of EEC were detected in biopsies and 7,22% in surgical pieces (p< 0.0002). The torpid evolution of the disease, its rapid progression and death was significantly greater in patients with HCC than in patients with EEC (34,43% vs.17,1%; P< 0.002), since HCC was diagnosed in more advanced stages and it had 19,12% adenocarcinoma and adenosquamoushistology vs. 8,37% in EEC.
Conclusions: patients with HCC had a worse evolution and pooroutcomesignificantly greater than in patients with EEC. The use of virological tests in the screening would allowto detect HCC in early stages
Key words
uterine cervix carcinoma
Introduction
Hidden cancer of uterine cervix is an endocervical and /or endoglandular carcinoma located deep or higher up in the miocervix, reason by which it constitutes a big diagnosis problem and a challenge for colposcopist and cytopathologist experts.
The revision of smears in patients with cervical carcinoma, demonstrated 4% of diagnostic doubts originated in reactive and inflammatory changes, and the presence of immature squamous metaplastic type cells (ISM) in 68,75%[1,2].
The squamous carcinomas originated in ISM that evolve deep in the endocervical canal and into de glandular crypt are very frequently, inaccessible to colposcopic and cito-histophatologic diagnosis, with delayed expression in advanced stages[3]. In 60% of ISM, HPVtypes 16 and 18 have been detected[4]. In the same way, the adenocarcinomas, specially the ‘endometroide’ variant, originated high up in the cervical canal evolves hidden or inaccessible to diagnostic methods. The latter is the most frequent type in the cervix[5].
Glandular or squamous lesions originated in glandular epithelium or ISM, into the cervical canal, 3 to 4 cm long, with cripts that reach up to 8-10 mm deep in the miocervix according to the authors, cause difficulties and delays in the diagnosis, since their evolution is hidden and thus are detected in more advanced stages. The chances of missing out the lesion are considerable[6,7]. Frequently, these are patients with negative smears and normal or low suspicion colposcopies, even in the last six to twelve months preceding a diagnosis of carcinoma [8,9].
The objectives were to evaluate the incidence of hidden cervical cancer (HCC), to analyze the diagnostic, histopathological aspects and clinical evolution.
Material and methods
In Gynecological Services of the National Hospital Prof. Dr. A. Posadas, between January 1995 and December 2013, 677 patients were diagnosed with cervical cancer.
Design: retrospective and observational study.
Unit of study: all women with cervical cancer who were attended during this period. A revision of their clinical cards and histories was made.
Selection criteria: patients with cervical cancer and a follow-up of more than twelve months. The tumor diagnosis and stage was assessed according to criteria of the Argentine Association of Gynecology & Oncology (AAGO), FIGO 2009 norms and staging and the histopathological criteria of the World Health Organization (WHO). Ectocervical and endocervical cytology with brush (brushing), and colposcopy of lower genital tract were performed. Histopathological study of all biopsies and surgical specimens was performed.
Patients received treatments according to the clinical staging of disease: total hysterectomy, radical hysterectomy with lymph node dissection, with removal of the fallopian tubes and ovaries (Wertheim-Meigs), or without removal fallopian tubes and ovaries, neoadyuvant chemotherapy, concurrent chemotherapy and radiotherapy, braquitherapy and telecobaltotherapy. A follow-up was held for more than twelve months.
Statistical analysis: Chi square, Fisher´s exact and probability test. The probability values <0.05 was considered significant.
Definition: hidden cancer of the uterine cervix (HCC) refers to the cancer located deep or up in the endocervical canal and miocervix, whose detection is delayed, with negative cytology, colposcopy and biopsies 6 to 12 months before its clinical and / or cyto-colposcopical expression; distinguishing it from the cancer located in the exocervix or exo-endocervix (EEC).
Results
The mean age of the patients with HCC was 47.5 years old (29-77) and the age of those who presented EEC was 48.7 years old (21-90). The hidden cervical carcinoma was found in 68/677 patients representing 10,04%.
Patients with HCC consulted mostly for a medical checkup: 55,88%, for bleeding: 33,82%, for sinusorragia: 19,1%, for hematometra and pyometra: 2,94%, and for the appearance of one inguinal node: 1,47%; whereas most patients with EEC consulted for bleeding (62%), for coital bleeding and vaginal discharge with odor (23%) and for a medical checkup only 16% (p<0.0003; Figures 7).
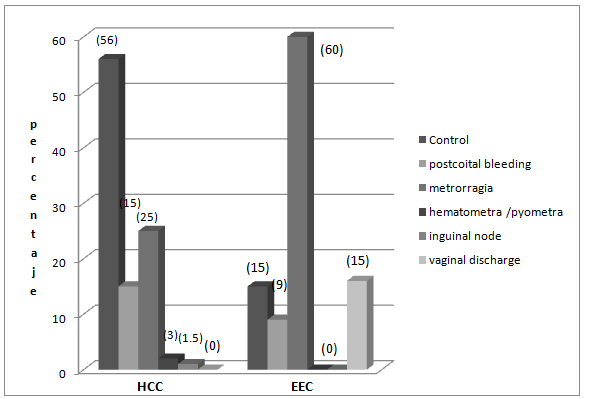
Figure 7. Reasons for consultation of patients with Hidden Cervical Carcinoma (HCC) and exocervical carcinoma (EEC).
The sensitivity of cytology for HCC was 47,06% (IC 95%, 35.2-59) with a 36,76% of false negatives and a 16,18% of subdiagnoses; whereas forexo-encocervical carcinoma (EEC) it was 69,13% (IC 95%, 65.4-72.8), 12,32% and 18,56% respectively (p<0,001; Table 1). ISM cells without atypia were found in 28 patients with HCC (41,2%).
Table 1. Cytological aspects.
|
CYTOLOGY |
HCC
n.pat. % |
EEC
n. pat. % |
False negatives |
25 * (36,76) |
75 (12,32) |
Subdiagnosis |
11 ** (16,18) |
113 (18,56) |
Suspected carcinoma |
32 (47,06) |
421 (69,13) |
Total |
68 (100) |
609 (100) |
HCC: Hidden cervical cancer, EEC: exo-endocervical carcinoma.
*Ascus :1, HPV: 1, ISM: 20; **ISM: 8, CIN II-III: 2
pat: patients
The colposcopy’s sensitivity for HCC was 17,65% (IC 95%, 8.6-26.7); whereas for EEC it was 85,55% (IC 95%, 82.8-88.4) (p<0.0001;Table 2).
Table 2. Colposcopic aspects
|
COLPOSCOPY |
HCC
n. pat. % |
EEC
n.pat. % |
Normal |
15 (22,06) |
-- |
Low suspicion |
41 (60,29) |
97 (15,93) |
Suspected cancer |
12 (17,65) |
512 (85,55) |
Total |
68 (100) |
609 (100) |
HCC: Hidden cervical cancer, EEC: exo-endocervical carcinoma
30,88% of HCC cases were diagnosed by biopsies and 41,18% through surgical procedures (LLETZ, CONE and hysterectomy); whereas 89,3% of EEC cases were detected by biopsies and 7,22% in surgical pieces (p<0.0002; Table 3). In these patients invasive carcinoma in advanced stages (E Ib to E IV) was detected: in 6 of them, in pieces of LLETZ located at more than 5 to 9 mm of deep ectocervix; in 2 patients, in the vertex of the CONE and in 3 of them, in pieces of hysterectomy performed because of other pathologies (pyometra, hematometra and ovarian blastoma); whereas 89,3% of EEC cases were detected by biopsies and only 7,22% in pieces of LLETZ and CONES (p<0.0002;Table 3, Figures 1-4).
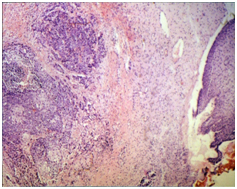
Figure 1. Microphotograph 100 X- Hematoxiline-Eosine (H&E). Patient with an inguinal lymph node with squamous carcinoma metastasis as the only symptomatology. She is referred to Gynecological Services. Negative cytology, colposcopy and anal examination. Increased consistency of cervix, regular and the transformation zone was not visible. Invasive squamous carcinoma found in the piece of LLETZ (stage IV).
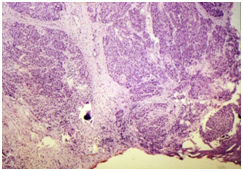
Figure 2. Microphotograph 40 X- H&E. Same patient as in figure 1. Thick carcinomatous cords invading the upper part of miocervix were observed.
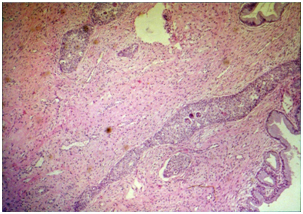
Figure 3. Microphotograph 40 X- H&E. Patient with conisation by carcinoma in situ. Insufficient cone by greater pathology in the vertex. Invasive squamous carcinoma with lymph vessels.
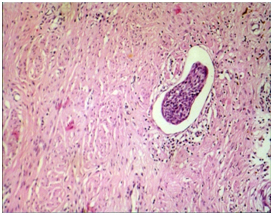
Figure 4. Microphotograph 400 X- H&E. Same patient as in figure 5, lymph embolism of squamous carcinoma in the piece of hysterectomy (WertheinMeigs).
Table 3. Diagnostic methods.
|
METHODS |
HCC
n.pat. % |
EEC
n.pat. % |
Biopsies |
21 (30,88) |
544 (89,33) |
ECC / D&C / HSC |
19 (27,94) |
21 (3,45) |
LLETZ |
13 (19,12) |
29 (4,76) |
Conisation |
12 (17,65) |
15 (2,46) |
Hysterectomy |
3 (4,41) |
-- |
Total |
68 (100) |
609 (100) |
HCC: Hidden cervical cancer, EEC: exo-endocervical carcinoma
ECC / D&C / HSC: endocervical curettage / endometrial curettage / hysteroscopy. LLETZ: Large Loop Excision Transformation Zone
Out of 68 patients with HCC, 55 of them (80,88%) had squamous carcinomas and 13 of them (19,12%) had adenocarcinomas (6/11 undifferentiated) and adenosquamous (1/2 undifferentiated); whereas in the EEC the squamous type was observed in 558/609 (91,63%) patients and adenocarcinomas in 51 patients (8,37%).The incidence of the glandular type was significantly more often in the HCC (p<0.0001Figures 5,6).
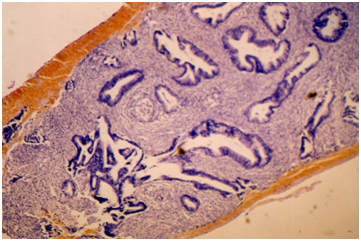
Figure 5. Microphotograph 40 X- H&E. Higher up in the endocervical canal asymptomatic adenocarcinoma was detected by curettage.
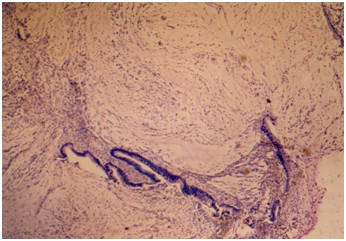
Figure 6. Microphotograph 40 X- H&E. Same patient as in figure 5, adenocarcinoma invading the isthmus was observed in the piece of subtotal hysterectomy for pyometra and hematometra (stage IV).
All patients with HCC attended the consult with more advanced stages than I/b at diagnosis, and 46/68 (67,65%) with more advanced stages than IIa/b at the moment of detection: Ib1: 22 (32,55%), IIa/b: 14 (20,59%), IIab: 12 (17,65%), III:14 (20,59%) y IV:6 (8,82%).
Among patients with HCC, 32 (47,06%) were treated surgically: a total of 3 hysterectomies with vaginal sleeve and 29 (42,65%) had radical hysterectomies with lymph node dissection performed, with or without removal of fallopian tubes and ovaries (Wertheim-Meigs). 12 (17,65%) of them had adjuvant radiation therapy and / or neoadyuvant chemotherapy for a more advanced surgical stage. Chemotherapy, concurrent chemotherapy and radiotherapy, braquitherapy and telecobaltotherapy were performed in 35 (51,47%) patients with non surgical stages (IIab, III y IV).
Follow-up
In 7/68:10,29% patients data was not registered because they were treated or their follow-up was carried out in other centers. Out of the 61 patients with HCC, 21 (34,43%) with advanced stages had a rapid progression of the disease and death: 9 (42,86%) of them in less than one year (2 patients with stage IIab, 4 with stage III and 3 with stage IV), 10 (47,62%) of them between one and two years (3 patients with stage IIab, 5 with stage III and 2 with stage IV) and 2 (9,52%)of them between two and three years (stages IIab and IV); whereas 104/ 609 (17.1%) patients with advanced stages of EEC had torpid evolution and death in less than three years (p<0.002).
Discussion
The HCC is originated deep in the endocervical canal, or into glandular crypts in the upper part of the canal, or in focal Immature Squamous Metaplasia (ISM). Its detection in advanced stages was most frequently observed by delays in the clinical expression [3,5].
The presence of small adenocarcinomas in absence of prior adenocarcinomas in situ, suggests the possibility of rapid progression[5]. Symonds P. et al.suggest that diagnosis in advanced stages is more dependent on the biological behavior of the tumor than on the delay in its clinical expression. However they observed, and it is noteworthy, that the diagnosis of asymptomatic invasive cervical cancer was made following a routine smears only in 5,7%10.
Most of the patients with HCC were asymptomatic (55,8%) and consulted for a medical checkup; whereas only 16% of the women with EEC were asymptomatic. These results match previous studies [9,10](p<0.0003).
The sensitivity of cytology for HCC was less than for EEC, and the number of false negatives and subdiagnosis was greater in the HCC patients; the differences were significant and match findings in previous studies[11,12].
In this study we found ISM cells without atypia in patients with HCC (41,2%). The finding of these ISM cells raises frequent doubts and errors in its interpretation[2,13]. Santos et al., found high-risk HPV types in 16% of women with ISM atypical cells[14].
In previous studies, we have observed by hibridation in situ, HPV types 16 and 18 in areas of mature and immature squamous metaplasia, near condilomas and Cervical Intraepithelial Neoplasia (CIN), associated with CIN II- III more frequently in the immature metaplasia than in mature metaplasia, where HPV types 6 and 11 predominated. Similar findings were observed by others autors[4,13,15].
The false-negatives cytologic smears, either because of inadequate sampling or because of diagnostic errors, are recognized causes of the delayed detection of the disease[16-19].
Therefore, it is necessary to consider the possible existence of a hidden carcinoma deep in the cervix and to optimize the diagnostic methodology, adding, as well, some of the virological tests that are already successfully being used in some developed countries[20-24]. Using exocervical cytology and endocervical brushing, the false-negatives decrease only 3% [25,26].
Hatem and Wilbur in the Rochester University Medical Center, related false-negative smears with rapid progression and they published the presence of atypical immature squamous metaplasia cells in 68%[3].
Al-Nafussiet al. observed that the cellular changes associated with inflammation, atrophy or mature squamous metaplasia have minor cervical predictive value for neoplasia, than those associated with immature squamous metaplasia[27].
Most of the patients with HCC displayed negative colposcopy or low suspicion, with delayed clinical expression. The HCC was missed on cytology smears as well as on colposcopically directed biopsies and was detected only on abrasive brushing and/ or endocervical curettage, LLETZ, conisation and hysterectomy. The difference with EEC was significant. Similar data was observed by others authors[28-30].
Kodiathodiet al., found a significant proportion of women with negative colposcopy and nuclear borderline changes in endocervical cells, and the presence of H-SIL and carcinomas during the first year of follow-up. They propose to optimize the algorithm diagnosis to detect high grade histology and cancer in earlier stages [31].
Although the squamous type in both HCC and EEC was most frequent in the cervix (80,88% y 91,63% respectively), adenocarcinomas and adenosquamous had a significantly greater incidence in HCC (19,12%) than in the EEC (8,37%). Similar issues match previous studies[32].
As regards the evolution of the disease, its rapid progression and death was significantly greater in patients with HCC than in patients with EEC (34,43% vs.17,1%; P<0.002), due to its presentation in advanced stages and its histologic type.These differences were related, not only to the stage of the tumor, advanced in most cases, but also to the histologic type (adenocarcinoma and adenosquamous: 19,12% in HCC and 8,37% in EEC) and its differentiation, since its biological behavior is more aggressive and with worse prognosis[10,33,34].
It is important to focus in early diagnosis of cervical cancer. Therefore, it is necessary to optimize the diagnostic methodology available, investigating risk factors for cervical cancer development and including virological tests that wouldallow to detect hidden endocervical lesions[20-24,35-37].
We consider of great importance to implement health policies that aim to prevention and early detection of cervical cancer. This will lead to improve prognosis of the disease, survival, quality of life and to significantly reduce therapeutic costs.
Conclusions
- The squamous and glandular lesions originated deep or in the crypts or in the upper part of the endocervical canal, evolve hidden, and are missed by the diagnostic methods. They are frequently asymptomatic and detected in advanced stages.
- Patients with HCC have a rapid progression and death significantly greater than in patients with EEC.
- To optimize the usual diagnostic methodology and to use virological tests in the screening, would allow to detect with greater success endocervical and endoglandular lesions, and the hidden carcinoma in earlier stages.
References
- Frisch LE1 (1987) Inflammatory atypia and the false-negative smear in cervical intraepithelial neoplasia. Acta Cytol 31: 873-877. [Crossref]
- Walker EM, Hare MJ, Cooper P (1983) A retrospective review of cervical cytology in women developing invasive squamous cell carcinoma. Br J Obstet Gynaecol 90: 1087-1091. [Crossref]
- Hatem F, Wilbur DC (1995) High grade squamous cervical lesions following negative Papanicolaou smears: false-negative cervical cytology or rapid progression. Diagn Cytopathol 12: 135-141. [Crossref]
- Dalbert D Tesis “Comparative study of HPV infection & intraepithelial neoplasie in the woman and her partner, & conservative treatment of the uterine cervix with diathermic large loop excision”.1994, Vol. II: 328-330. Bibliotheque of Medicine.University of Buenos Aires Argentine.
- Lee KR, Flynn CE (2000) Early invasive adenocarcinoma of the cervix. Cancer 89: 1048-1055. [Crossref]
- Anderson MC, Hartley RB (1980) Cervical crypt involvement by intraepithelial neoplasia. Obstet Gynecol 55: 546-550. [Crossref]
- Saffe I, Marone S (1983) Posible relación entre la longitude de las criptas endocervicales y los fracasos de la crioterapia en el tratamiento del carcinoma in situ pavimentoso del cuello. Obstet Gynaecol Latino-Am.
- Mitchell H, Medley G, Giles G (1990) Cervical cancers diagnosed after negative results on cervical cytology: perspective in the 1980s. BMJ 300: 1622-1626. [Crossref]
- Deshpande AH, Munshi MM (1998) Diagnostic dilemma in high intracanalar carcinoma of the cervix. Indian J Cancer 35: 107-111. [Crossref]
- Symonds P, Bolger B, Hole D, Mao JH, Cooke T (2000) Advanced-stage cervix cancer: rapid tumour growth rather than late diagnosis. Br J Cancer 83: 566-568. [Crossref]
- Bartt O, Mural J, Dalbert D, Rodriguez de la Peña M, Sandoval A, Borelli A (1996) Invasive Carcinoma of uterine cervix. First International Congress of Low Genital Tract Patology & Colposcopy. XXIV Annual American Meeting. 84 Summary Book.
- Bartt O, Mural J, Dalbert D, Sandoval AP, Borelli, R. de La Peña M (1996) Invasive Carcinoma of uterine cervix upgrade. Presented in the XIV Obstetric & Gynecology Jornada. Obstet & Gynecol Society Bs Aires, Argentine. G-56, Summary Book.
- Wilbur DC (1997) False negatives in focused rescreening of Papanicolaou smears: how frequently are 'abnormal' cells detected in retrospective review of smears preceding cancer or high-grade intraepithelial neoplasia? Arch Pathol Lab Med 121: 273-276. [Crossref]
- Santos AL, Derchain SF, Martins MR, Sarian LO, Martinez EZ, et al. (2003) Human papillomavirus viral load in predicting high-grade CIN in women with cervical smears showing only atypical squamous cells or low-grade squamous intraepithelial lesion. Sao Paulo Med J 121: 238-243. [Crossref]
- Distéfano AL, Picconi MA, Alonio LV, Teyssié A, Dalbert D, et al (1998) Persistence of Human Papillomavirus DNA in Cervical Lesions after treatment with Diathermic Large Loop Excision. Rev Infectious Disease in Obstet and Gynecology 6: 214-219.
- Coleman DV, Poznansky JJ (2006) Review of cervical smears from 76 women with invasive cervical cancer: cytological findings and medicolegal implications. Cytopathology 17: 127-136. [Crossref]
- Pereira ER, Speck NM, Rodrigues DA, De Freitas VG, Ribalta JC (2015) Prevention, diagnosis and treatment of cervical cancer precursor lesions at the Xingu Indigenous Park, Brazil. Eur J Gynaecol Oncol 36: 376-382. [Crossref]
- Mubiayi N, Bogaert E, Boman F, Leblanc E, Vinatier D, et al. (2002) Cytological history of 148 women presenting with invasive cervical cancer. Gynecol Obstet Fertil 30: 210-217. [Crossref]
- Repše-Fokter A, Pogacnik A, Snoj V, Primic-Žakelj M, Fležar MS (2012) Review of negative and low-grade cervical smears in women with invasive cervical cancer after the first 3 years of the national cervical screening programme in Slovenia. Cytopathology 23: 23-29. [Crossref]
- Castle PE, Solomon D, Schiffman M, Wheeler CM (2005) Human papillomavirus type 16 infections and 2-year absolute risk of cervical precancer in women with equivocal or mild cytologic abnormalities. J Natl Cancer Inst 97: 1066-1071. [Crossref]
- Szarewski A, Ambroisine L, Cadman L, Austin J, Ho L, et al. (2008) Comparison of predictors for high-grade cervical intraepithelial neoplasia in women with abnormal smears. Cancer Epidemiol Biomarkers Prev 17: 3033-3042. [Crossref]
- Monsonego J, Pintos J, Semaille C, Beumont M, Dachez R, et al. (2006) Human papillomavirus testing improves the accuracy of colposcopy in detection of cervical intraepithelial neoplasia. Int J Gynecol Cancer 16: 591-598. [Crossref]
- Wang JL, Yang YZ, Dong WW, Sun J, Tao HT, et al. (2013) Application of human papillomavirus in screening for cervical cancer and precancerous lesions. Asian Pac J Cancer Prev 14: 2979-2982. [Crossref]
- Carozzi FM, Iossa A, Scalisi A, Sideri M, Andersson KL, et al. (2015) hr-HPV testing in the management of women with ASC-US+ and in the follow-up of women with cytological abnormalities and negative colposcopy. Recommendations of the Italian group for cervical cancer screening (GISCi). Epidemiol Prev 39: 84-90. [Crossref]
- Andersen W, Frierson H, Barber S, Tabbarah S, Taylor P, et al. (1988) Sensitivity and specificity of endocervical curettage and the endocervical brush for the evaluation of the endocervical canal. Am J Obstet Gynecol 159: 702-707. [Crossref]
- Redondo Horcajo AM, Guerra Merino A, Pinedo Garrido G, García Aranda R (2000) Prevention of cervix cancer. Comparison of the sample quality obtained using cotton swab or cervical brush. Aten Primaria 26: 38-41. [Crossref]
- al-Nafussi A, Rebello G, al-Yusif R, McGoogan E (2000) The borderline cervical smear: colposcopic and biopsy outcome. J Clin Pathol 53: 439-444. [Crossref]
- Lynge E, Arffmann E, Poll P, Anderson PK (1993) Smear misclassification in a cervical cancer screening programme. Br J Cancer 68: 368-373. [Crossref]
- Shingleton HM, Gore H, Austin JM, Littleton HJ, Straugin JM (1975) The contribution of endocervical smears to cervical cancer detection. Acta Cytol 19: 261-264. [Crossref]
- Hatch KD, Shingleton HM, Orr JW Jr, Gore H, Soong SJ (1985) Role of endocervical curettage in colposcopy. Obstet Gynecol 65: 403-408. [Crossref]
- Kodiathodi S, Chattopadhyay S2, Baldwin A1, Franks P1 (2014) Management of borderline change in endocervical cells: a more dependable approach. Br J Cancer 111: 851-857. [Crossref]
- Mural J, Borelli MA, Rodriguez de la Peña M, Marrone M, Stigliano J (2012) Uterine cervical cancer incidence in women minors of 35 years. Rev Argentine Association of Gynecology & Oncology (AAGO) 1-8.
- Farley JH, Hickey KW, Carlson JW, Rose GS, Kost ER, et al. (2003) Adenosquamous histology predicts a poor outcome for patients with advanced-stage, but not early-stage, cervical carcinoma. Cancer 97: 2196-2202. [Crossref]
- Huang YT, Wang CC, Tsai CS, Lai CH, Chang TC, et al. (2012) Clinical behaviors and outcomes for adenocarcinoma or adenosquamous carcinoma of cervix treated by radical hysterectomy and adjuvant radiotherapy or chemoradiotherapy. Int J Radiat Oncol Biol Phys 84: 420-427. [Crossref]
- Choi JW, Kim Y, Lee JH, Kim YS (2016) The clinical performance of primary HPV screening, primary HPV screening plus cytology cotesting, and cytology alone at a tertiary care hospital. Cancer Cytopathol 124: 144-152. [Crossref]
- Julia C Gage, Mark Schiffman, Hormuzd A Katki, Philip E Castle, Barbara Fetterman, et al. (2014) Reassurance Against Future Risk of Precancer and Cancer Conferred by a Negative Human Papillomavirus Test. JNCI J Natl Cancer Inst 106: dju153
- Schiffman M, Wentzensen N, Wacholder S, Kinney W, Gage JC, et al. (2011) Human papillomavirus testing in the prevention of cervical cancer. J Natl Cancer Inst 103: 368-383. [Crossref]







