Objectives: The goal of the study was to evaluate a transmucosal gingival patch (PerioPatch®) containing a combination of botanical extracts. The PerioPatch has demonstrated a clinical reduction of inflammation and the promotion of healing by reducing inflammatory exudates. The PerioPatch has also been found to reduce both cellular matrix metalloproteinase (MMP) production and overall MMP activity. A proof-of-concept clinical study was conducted to determine the effect of PerioPatch on tissue MMP-9 levels and gingival inflammation.
Methods: Dry hydrogel patches (PerioPatch) containing a proprietary botanical extract blend were applied to inflamed gingival tissue. Twenty patients scheduled to undergo periodontal surgery were randomized for treatment with active or placebo patches 24 hours prior to surgery. Gingival inflammation was measured at baseline and 24 hours later, immediately prior to surgery. Tissue levels of MMP-9 were measured in biopsy samples taken during surgery. In-vitro assays were also performed to determine the effect of PerioPatch on the activity of purified MMP-2 and MMP-9. Fibroblast production of tissue inhibitor of metalloproteinase 3 (TIMP3) activity was also measured by using reverse zymography, as was absorptive capacity of PerioPatch.
Results: There was a significant decrease in tissue levels of MMP-9 within 24 hours after topical administration of the test treatment, as compared to placebo. In a separate study, PerioPatch was shown to have increased absorptive capacity when compared to placebo patches. In the cell-free assay, the enzymatic activity of MMP-2 and of MMP-9 was directly reduced by the PerioPatch botanical blend. In addition, fibroblasts exposed to the PerioPatch were induced to produce TIMP3.
Conclusions: This pilot clinical study, which was supported by in-vitro assay results, demonstrates that PerioPatch can reduce inflamed gingiva and enhance tissue healing by absorbing inflammatory exudates, resulting in a reduction of MMP activity and an induction of TIMP3 activity.
More than 80% of people over the age of 65 years living in the United States suffer from periodontitis [1]. Periodontal diseases are bacterially induced, chronic inflammatory conditions that lead to destruction of soft and hard tissue supporting the teeth. Current understanding of the pathogenesis of periodontitis has shifted away from viewing the bacterial plaque as the primary factor driving disease, to a host-response centered view. The complex immuno-inflammatory response to bacterial plaque, together with genetic and environmental factors that modulate response, is ultimately responsible for disease outcome [2].
Despite this paradigm shift, currently available therapeutic options for managing the host immuno-inflammatory response in the oral cavity are limited. Low-dose doxycycline requires systemic administration and long-term therapy (>3 months) to reduce matrix metalloproteinase (MMP) levels and to effect changes in the periodontal tissue [3]. Topical anti-inflammatory agents, such as COX-inhibitors, have been shown to irritate mucosal tissue [4], and repeated application of topical steroids raises the risk of infection [5] and reduces the capacity for normal tissue repair. The absence of safe topical agents to control oral mucosal inflammation stands in stark contrast to the modalities available to manage inflammation when present in other topically accessible sites such as the skin, eyes, or rectum.
The tissue destruction that results from gingival inflammation is induced by host enzymes that damage and degrade the extracellular matrix (ECM). Chief among these enzymes are the MMPs, which are zinc-dependent endopeptidases capable of degrading the collagen and elastin of the ECM. MMP expression is activated by zinc-dependent pro-inflammatory cytokines, such as IL-1β, all of which are involved in the progression of periodontitis [6,7]. The proteolytic activity of MMPs is regulated by tissue inhibitors of metalloproteinases (TIMPs) [8,9] so that remodeling and repair can occur [10]. In pathological states of periodontal inflammation, many studies have shown that the balance between MMPs and TIMPs is disrupted, resulting in increased MMP activity [6,11-13]. MMP-1, -2, -3, -4, -7, -8, -9, and -13 have been detected in the gingival tissue or crevicular fluid of patients suffering from periodontal disease [14]. Significantly, the tissue or crevicular levels of MMPs increase in experimental gingivitis [15] and decrease in response to periodontal therapy or treatment with low-dose doxycycline [6,16].
Mode of Action
In general, the activity of wound dressings is based on their physical action, such as protection of inflamed tissue or absorption of fluids from inflammatory sites. This mechanical action can generate physiological changes in the tissue that can be demonstrated clinically and quantified in in-vitro assays.
PerioPatch
Among them, PerioPatch (Izun Pharmaceuticals, http://izunpharma.com) is a hydrogel wound dressing for use in the oral cavity. It is intended for the management of all types of oral wounds, injuries, and ulcerations of the gingiva and oral mucosa. They include stomatitis, minor chafing, and traumatic ulcers, as well as abrasions caused by orthodontic appliances and ill-fitting dentures, and lesions associated with oral surgery. The patch is self-adhesive after contact with the moist tissue of the oral cavity and provides an absorbent and flexible barrier over the affected area. As such, the patch protects inflamed areas and lesions in the mouth from contact with the rest of the oral environment, thereby preventing irritation and painful aggravation of the affected area. The patch absorbs the inflammatory enzymes and allows for natural resolution of the inflammation. This action has been observed clinically, as well as in in-vitro assays not yet published.
The primary effect of PerioPatch is therefore achieved by physical means, i.e., removal of toxic exudates and protection of healing tissue. There are two aspects of PerioPatch that influence the absorption of exudates: patch adhesion and the absorptive capacity of the patch. It has also been demonstrated in unpublished studies that the adhesive properties of the basic patch are enhanced by the inclusion of the botanical compounds into the patch. A series of in-vitro experiments were performed, and results indicate that the patch, in the absence of the botanical ingredient, absorbs up to 40% of its initial weight in aqueous fluid; the same patch containing the botanical ingredient absorbs an average 70% of its initial weight in aqueous fluid (Figure 1). This double absorption effect, that of the patch itself augmented by that of the botanical ingredient, is essential for the effective removal of exudates secreted during an inflammatory response of the gingiva.
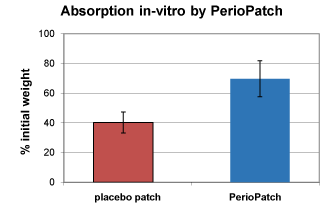
Figure 1. In-vitro absorption of fluids by PerioPatch. Note a close to doubling of absorptive capacity upon inclusion of botanical extracts in PerioPatch when compared to that of the placebo, an identical patch lacking the botanical ingredient.
Background: Botanical Extracts and the Inflammatory Response
Botanical extracts are a known source of bioactive compounds that can inhibit or exacerbate the inflammatory response [17]. They have also been shown to aid in absorption of pro-inflammatory mediators and, as such, support the natural resolution of the inflammation. Since extracts contain multiple chemical compounds, careful evaluation of their effects in preclinical models and in controlled clinical trials is critical. It is believed by researchers involved in the development of PerioPatch that this treatment could provide a safe and effective means of controlling gingival inflammation topically, providing a needed adjunct for the management of periodontal disease.
Study Objectives
A randomized, placebo-controlled, proof-of-concept clinical trial was conducted to evaluate the capacity of topical treatment with PerioPatch to reduce tissue MMP levels. This study also investigated whether MMP-2 and MMP-9 activity could be inhibited by a combination of Echinacea purpurea, Centella asiatica, and Sambucus nigra, formulated into an absorptive matrix. In addition, fibroblasts were exposed to PerioPatch plant extracts to establish whether the production of the MMP inhibitor TIMP3 could be induced.
PerioPatch matrix
Ethanol/water extracts of the plants C. asiatica, E. purpurea and S. nigra were produced in conformance with European Pharmacopeia standards for analysis and purity, and ultimately incorporated into a dry hydrogel matrix that adheres and conforms to gingival tissue.
Gelatin zymography
These experiments were based on gelatin zymography, a cell-free assay whereby 0.06 µg of human MMP-2/9 standards (Chemicon International, www.chemicon.com) were incubated with increasing concentrations of the extract, ranging from a total of 0.125 mg/ml to a total of 2 mg/ml of extract for 1 hour at 37ºC. Samples were mixed with Novex TRIS-Glycine SDS Sample Buffer (2X) (ThermoFisher Scientific, www.thermofisher.com) at a dilution factor of 1/100 and loaded onto Novex 10% Zymogram (0.1% gelatin) Gels. Electrophoresis was carried out at a constant current of 40mA and a constant voltage of 125V for 2 hours. Novex TRIS-Glycine SDS Running Buffer (10X) was also used as the electrophoresis buffer. After electrophoresis, the gels were soaked in 2.7% Triton X-100 made up in ddH2O for 2 hours and incubated at 37ºC overnight in Novex Developing Buffer (10X). The gels were stained for 1 hour in Brilliant Blue R (Sigma-Aldrich, http://www.sigmaaldrich.com), and destained for 2 hours in 10% glacial acetic acid prepared in 30% methanol. Enzyme activity was quantified using an F-ChemiBIS 1.6 gel documentation system (DNR Bio-Imaging Systems, http://dnr-is.com). All Novex products mentioned above were obtained from Invitrogen (ThermoFisher Scientific).
Reverse zymography
Tissue Inhibitor of Metallo-Proteinase 3 (TIMP3) activity was assayed using reverse zymography with a Reverse Zymography Kit developed by Dylan Edwards, PhD, University of East Anglia, UK. HLF fibroblasts were seeded at a density of 2.5 x 105 cells/well in six-well plates and cultured for 24 hours. Growth medium was replaced with starvation medium (DMEM 4mM glutamine, 1 mM sodium pyruvate, 100 IU/mL penicillin, 0.1 mg/mL streptomycin, and 0.1% BSA) with or without transforming growth factor-beta (TGF-β; R&D Systems, www.rndsystems.com ) or test extract for an additional 24 hours. Conditioned medium was removed from each well, vacuum-dried, and re-suspended in 400 μl of 0.1% SDS. Samples were electrophoresed on a 12% SDS-PAGE gel that incorporated gelatin and recombinant MMP-2. The gels were then washed in rinse buffer (50 mM Tris, 5 mM CaCl2, and 25 g Triton-X 100) for 2.5 hours, followed by incubation buffer (rinse buffer without Triton-X 100) at 37°C overnight. The gels were stained in Coomassie Blue (Bio-Rad) for 2.5 hours and destained until a bright blue band was obtained against a clear background. The image was captured with a CCD camera (DNR Bio-Imaging Systems). Densitometry was performed with TotalLab software (Nonlinear Dynamics, http://www.nonlinear.com).
Absorption by PerioPatch
PerioPatch samples were weighed, using an analytical balance. Measured volumes of ¼N saline were applied to the adhesive surface of the patch sample, which was then incubated for 60 minutes at 37ºC, 80% relative humidity. After incubation, excess saline was carefully removed using Whatman filter paper (qualitative #1), and the weight of each sample before and after saline absorption was calculated and compared to that of a placebo patch containing no botanicals.
Patient population
Twenty patients between 18 and 75 years of age who were scheduled for periodontal surgery were enrolled in a placebo-controlled, matched-sample study to determine whether topical treatment with a dry hydrogel patch containing a blend of E. purpurea, C. asiatica, and S. nigra extracts would result in a decrease in the tissue levels of relevant MMPs. Trial patients had similar periodontal disease conditions bilaterally and were scheduled to undergo two separately scheduled periodontal surgeries on opposite sides of the mouth within 3 to 4 weeks of each other. Subjects had undergone several courses of routine periodontal treatment consisting of scaling and root planing prior to the decision to proceed surgically. Acceptance criteria included gingival index (GI) scores greater than 1, as assessed by the method of Loe and Sillness [18], and at least two quadrants with three or more periodontal pockets between 5 mm and 12 mm.
Procedures
Treatment with the experimental patch was compared to treatment with a similar placebo patch that did not contain the botanical extracts. The patch was applied to the buccal aspect of the gingiva three times during the 24 hours preceding the surgical procedure. The first patch was applied by a periodontist, and the following applications were performed by the patient according to instructions from the periodontist.
Patients’ GIs were measured before placement of the first patch and 6 to 8 hours after placement of the third patch at the time of the periodontal surgery, at which time tissue biopsies were taken. Both the patient and the individual who performed clinical site assessment and evaluation of tissue samples were blinded as to whether the patch was the test or the placebo patch.
Gingival biopsy specimens were obtained from the buccal side (site of the patch) and the palatal side, with the palatal site used to assess whether the patch had an impact at tissue sites distant from the patch itself. Excised tissue was frozen and stored until analyzed. After a washout period of at least 3 weeks, the same procedure was repeated on the second side. Although the patch’s identity was known to neither the examiner nor the patient, the initial patch for all subjects was placebo, with the second patch for all subjects being the PerioPatch. The reason for this was to control for the possible therapeutic effect of the PerioPatch on the contralateral side. Approval to perform the study was obtained from the Institutional Review Board of Shaare Zedek Medical Center (Jerusalem, Israel), and all participating subjects provided informed written consent before the start of the study.
Determination of MMP-9 Levels in Biopsy Samples
Gingival biopsy specimens were homogenized in PBS, and the concentration of protein per sample was determined by Bradford assay using bovine serum albumin as a standard. Metalloproteinase activity was assayed by gelatin zymography, using 15% gelatin-containing gels as described above. Samples were applied to the gels under non-reducing conditions without heating. Gels were then soaked in 200 ml of 2% Triton X-100 in distilled water on a gyratory shaker for 30 minutes at 20ºC. They were then incubated in developing Tris buffer, pH 8.0, for 15 hours at 37ºC. Finally, gels were stained and washed according to a protocol described previously by Korostoff et al in 2000.19 Gels were photographed and scanned, and activity was measured using NIH Image (http://rsb.info.nih.gov/nih-image). For purposes of comparison of the same gel, one sample band was arbitrarily established as having a value of 1 (Group 3), and the band values from the other samples were calculated relative to that sample based on densitometric measurements of the clear band areas.
Statistical Analysis
In terms of clinical evaluation of periodontitis, GI scores were compared before and after treatment with the patch using McNemar's non-parametric test [20] to analyze the nominal data. To confirm the robustness of the analysis, Wilcoxon Signed rank test [21] was applied, as the ordinal scale for GI is limited, and the odds ratio was then calculated. To test for differences in the in-vivo assays for MMP-9, the Fisher Exact test [22] was applied because the sample size was small.
Absorption by PerioPatch
As can be seen in Figure 1, addition of the botanical ingredient to the patch formulation almost doubled the absorptive capacity of the patch.
Direct inhibition of enzyme activity
To measure the inhibitory properties of botanical extracts, different dilutions of extracted PerioPatch matrix (PPM) were mixed with gelatinase zymography standards of human MMP-2/9 for 1 hour and electrophoresed on gelatin gels. The resulting zymograms showed a concentration-dependent reaction where, at the highest concentration of 2 mg/ml of PPM, the MMP-2 activity was reduced to 28% that of the control, while MMP-9 activity was almost completely inhibited. At this extract concentration, the pro-MMP-9 activity was reduced to 34% of the control (Figure 2).
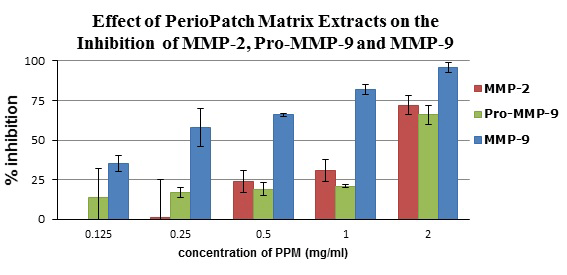
Figure 2. Inhibition of metalloproteinases MMP-2, MMP-9, and Pro-MMP-9 by PPM extracts, as measured on gelatin zymography gels. Note a concentration-dependent reaction where, at the highest concentration (2 mg/ml of PPM extract), the MMP-2 activity was reduced by 78%, while MMP-9 activity was almost completely inhibited. At this concentration of extract, the pro-MMP-9 activity was reduced by 66%.
Induction of fibroblast TIMP3 production
To confirm induction of TIMP3 by PPM, fibroblasts were incubated with the matrix extract, with or without TGF-β added to the growth medium. The induced protein was visualized using a reverse zymogram technique. The treatment of gingival fibroblasts with PPM induced production of active TIMP3 markedly more than did induction with TGF-β. At a concentration of 1.5 mg/ml, the extract was two- to three-fold more effective as an induction agent than was TGF-β and, at even lower extract concentrations, the generation of TIMP3 exceeded that induced by TGF-β alone (Figure 3).
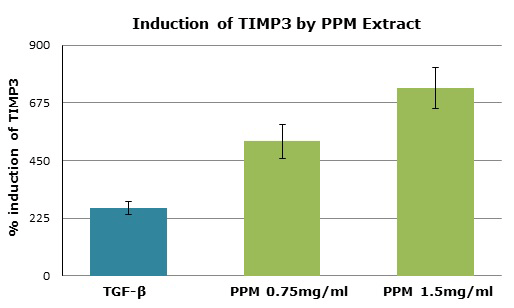
Figure 3. TIMP3 induction in HLF fibroblasts incubated with or without PPM extract and measured by reverse zymography. The treatment of gingival fibroblasts with the PPM extracts induced production of active TIMP3 markedly more than did induction with TGF-β. At a concentration of 1.5 mg/ml, the extract was two- to three-fold more effective as an induction agent than was TGF-β and, at even lower concentrations of PPM extract, the generation of TIMP3 exceeded that induced by TGF-β alone.
Treatment with extract-impregnated patch reduces GI
In terms of safety, there were no serious adverse events reported in this proof-of-concept study, which included 20 patients scheduled for periodontal surgery who were undergoing biopsies. The total of five adverse events reported by four patients were mild in severity. GI scores were obtained in the area where the patch was placed, and an average score was given per visit. A statistical comparison of the GI scores assessed at the area of the placebo and the PerioPatch were significantly different (P = 0.039). The odds ratio was 2.077 (95% confidence interval 1.035 - 4.384), confirming that PerioPatch had a positive effect in lowering GI scores (Figure 4).
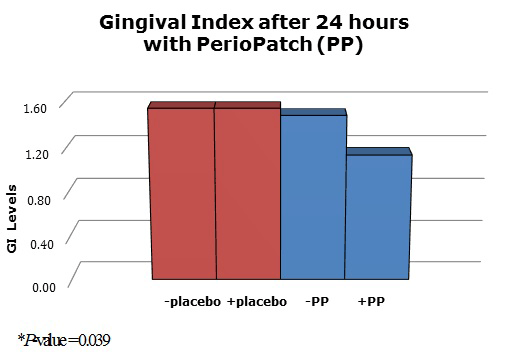
Figure 4. Gingival index of 20 patients before and after treatment with a placebo patch or PerioPatch. A statistical comparison of the GI scores assessed at the area of the placebo and of the PerioPatch were significantly different (P = 0.039). The odds ratio was 2.077 (95% confidence interval 1.035 - 4.384), confirming that PerioPatch had a positive effect in lowering GI scores.
Application of extract-impregnated patch reduces MMP-9 in vivo
MMP-9 protease activity was measured in biopsy specimens obtained from subjects prior to surgery. Overall, 78.6% biopsy samples in which PerioPatch was placed had less MMP-9 activity than the areas where the placebo patch was placed. The results of the enzyme assays were analyzed using two different statistical tests. The Wilcoxon signed rank test [21] was used as a non-parametric test for the case of two related samples or repeated measurements on a single sample. A significant statistical difference was seen in the results of the MMP-9 assay between those samples collected after application of PerioPatch and the samples obtained after the placebo patches had been on for 24 hours (P = 0.017). Similarly, a Fisher Exact test, [22] a contingency table test for small samples, showed that PerioPatch significantly inhibited the production of MMP-9 (P = 0.028) while, for biopsy material taken after the application of the placebo, no inhibition of the enzyme was observed (Figure 5).
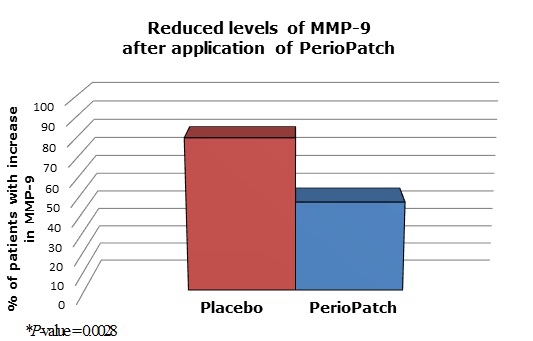
Figure 5. MMP-9 levels in patients treated with placebo or with PerioPatch. MMP-9 protease activity was measured in biopsy specimens obtained from subjects prior to surgery. A Fisher Exact test showed that PerioPatch significantly inhibited the production of MMP-9 (P = 0.028) while, for biopsy material taken after the application of the placebo, no inhibition of the enzyme was observed.
Oral pathogens are capable of triggering tissue destruction by inducing the host cells to increase release of matrix metalloproteinase (MMP), which is one of the mechanisms of indirect tissue destruction and tooth loss. Matrix metalloproteinases are zinc-dependent endopeptidases predominantly derived from polymorphonuclear leucocytes during acute stages of periodontal disease and are the key enzymes responsible for extracellular collagen matrix degradation. A significant increase in MMP-9 enzyme activity and TIMP induction has been reported in patients suffering from inflamed gingival disease [23].
In this proof-of-concept study, PerioPatch had a dramatic effect on the gingival index of the individuals in the study group. PerioPatch was applied three times over a 24-hour period and, on visual inspection of the gingiva, the signs of periodontal disease had abated in many of the patients. A placebo patch that had been applied in a similar manner 3 weeks earlier had had no effect on the GI. The etiology of the inflammation had already been previously removed, and the tissues were in a stagnated, chronically inflamed state; yet, with PerioPatch, there were substantial positive changes. The rapid improvement of the inflammatory lesions, as measured by the reduction in GI within a 24-hour period, was significant.
The gingival biopsies taken from both the buccal and palatal sides were analyzed for MMP-9 activity after application of both the placebo patch and PerioPatch. In the biopsies obtained after the treatment with PerioPatch, MMP-9 activity was sharply reduced in a majority of the samples. The major effect of PerioPatch appears to be its ability to remove pro-inflammatory exudates from the affected area, with the botanical ingredient greatly enhancing this effect.
An additional effect of the botanicals can be observed in the in-vitro study, wherein direct inhibition of the two gelatinases, MMP-2 and MMP-9, was achieved by incubating the enzymes with PPM. The highest inhibition was observed when 2 mg/ml of PPM was used, resulting in 96% and 72% reduction in MMP-9 and MMP-2 enzyme activity, respectively. PPM inhibited the enzyme activity in a concentration-dependent manner.
TIMPs regulate the activities of MMPs, and an imbalance between MMPs and TIMPs results in the pathological tissue destruction observed in periodontitis. The constitutive expression and evolutionary conservation of TIMP3 indicate its important function. TIMP3 induction by the cytokine TGF-β suggests that the role of this factor and TIMP3 in cartilage remodeling has important implications for periodontal disease. Induction of this natural inhibitor to improve the imbalance would aid the healing process. In this study, PPM induced TIMP3 far more efficiently than did TGF-β, even at the lower concentration of 0.75mg/ml of test material, demonstrating yet another effect of this patch.
Long-term longitudinal studies of patients with periodontal disease demonstrate that, despite receiving constant maintenance therapy, they suffer from continued periodontal attachment loss in areas of persistent inflammation [24,25]. This clearly indicates the need for additional modalities of therapeutic intervention to control the inflammatory process. PerioPatch could be used to augment current therapies, as well as to improve treatment outcomes and the success of long-term maintenance therapy. In addition, it has a strong role to play in supportive therapy in any indication where reducing inflammation and localized tissue destruction through the MMP pathway would improve the clinical situation or assist in patient management.
While inflammation is necessary to initiate repair of injured tissue, when dysregulated, it can damage tissue and prevent normal healing. Thus, the effective management of inflammation such as that demonstrated in chronic inflammatory disease—including gingivitis and periodontitis—is critical to promote healing. This is particularly the case after surgical procedures such as implant placement, periodontal surgery, ridge augmentation, and extractions.
The available topical approaches to managing inflammation in the oral environment have been associated with adverse events, including mucosal tissue irritation, increased risk of infection, and reduction in normal tissue repair. In contrast, the topical herbal patch described (PerioPatch) demonstrated reduction of inflammatory cell activity in the crevicular fluid and significant reduction of the MMP activity in the gingiva—all without serious adverse events.
This clinical trial suggests that botanical extracts offer a promising approach to topically reducing inflammation and promoting healing after surgical procedures and may have numerous additional applications in oral care as well.
- Albandar JM (2005) Epidemiology and risk factors of periodontal diseases. Dent Clin North Am 49: 517-532, v-vi. [crossref]
- Kinane DF, Bartold PM (2007) Clinical relevance of the host responses of periodontitis. Periodontol 2000 43: 278-293. [crossref]
- Fisher DA (1995) Adverse effects of topical corticosteroid use. West J Med 162: 123-126. [crossref]
- Reddy MS, Geurs NC, Gunsolley JC (2003) Periodontal host modulation with antiproteinase, anti-inflammatory, and bone-sparing agents. A systematic review. Ann Periodontol 8: 12-37. [crossref]
- Brewer AR, McCarberg B, Argoff CE (2010) Update on the use of topical NSAIDs for the treatment of soft tissue and musculoskeletal pain: a review of recent data and current treatment options. Phys Sportsmed 38: 62-70. [crossref]
- Sorsa T, Tjäderhane L, Salo T (2004) Matrix metalloproteinases (MMPs) in oral diseases. Oral Dis 10: 311-318. [crossref]
- Sorsa T, Tjäderhane L, Konttinen YT, Lauhio A, Salo T, et al. (2006) Matrix metalloproteinases: contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann Med 38: 306-321
- Varghese S (2006) Matrix metalloproteinases and their inhibitors in bone: an overview of regulation and functions. Front Biosci 11: 2949-2966. [crossref]
- Brew K, Dinakarpandian D, Nagase H (2000) Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta 1477: 267-283. [crossref]
- Mancini A, Di Battista JA (2006) Transcriptional regulation of matrix metalloprotease gene expression in health and disease. Front Biosci 11: 423-446. [crossref]
- Hannas AR, Pereira JC, Granjeiro JM, Tjäderhane L (2007) The role of matrix metalloproteinases in the oral environment. Acta Odontol Scand 65: 1-13. [crossref]
- Verstappen J, Von den Hoff JW (2006) Tissue inhibitors of metalloproteinases (TIMPs): their biological functions and involvement in oral disease. J Dent Res 85: 1074-1084. [crossref]
- Reynolds JJ, Meikle MC (1997) The functional balance of metalloproteinases and inhibitors in tissue degradation: relevance to oral pathologies. J R Coll Surg Edinb 42: 154-160. [crossref]
- Salvi GE, Lang NP (2005) Host response modulation in the management of periodontal diseases. J Clin Periodontol 32 Suppl 6: 108-129. [crossref]
- Lorencini M, Silva JA, de la Hoz CL, Carvalho HF, Stach-Machado DR (2009) Changes in MMPs and inflammatory cells in experimental gingivitis. Histol Histopathol 24: 157-166. [crossref]
- Ryan ME (2002) Clinical applications for host modulatory therapy. Compend Contin Educ Dent 23: 1071-1076, 1079-80, 1082. [crossref]
- Bisset NG. Herbal Drugs and Phytopharmaceuticals. Boca Raton: CRC Press; 1994.
- Löe H (1967) The Gingival Index, the Plaque Index and the Retention Index Systems. J Periodontol 38: Suppl:610-616. [crossref]
- Korostoff JM, Wang JF, Sarment DP, Stewart JC, Feldman RS, et al. (2000) Analysis of in situ protease activity in chronic adult periodontitis patients: expression of activated MMP-2 and a 40 kDa serine protease. J Periodontol 71: 353-360. [crossref]
- McNEMAR Q (1947) Note on the sampling error of the difference between correlated proportions or percentages. Psychometrika 12: 153-157. [crossref]
- NEW 21 (1945) Wilcoxon F Individual comparisons by ranking methods. Biometrics Bulletin. 1: 80-83.
- Zar JH (1999) Biostatistical Analysis. (4th edn.) Englewood Cliffs: Prentice-Hall.
- Ejeil AL, Igondjo-Tchen S, Ghomrasseni S, Pellat B, Godeau G, et al. (2003) Expression of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) in healthy and diseased human gingiva. J Periodontol 74:188-195. [crossref]
- Tonetti MS, Claffey N; European Workshop in Periodontology group C (2005) Advances in the progression of periodontitis and proposal of definitions of a periodontitis case and disease progression for use in risk factor research. Group C consensus report of the 5th European Workshop in Periodontology. J Clin Periodontol 32 Suppl 6: 210-213. [crossref]
- Lindhe J, Nyman S (1984) Long-term maintenance of patients treated for advanced periodontal disease. J Clin Periodontol 11: 504-514. [crossref]





