Aortic dissection is a relatively uncommon and potentially misdiagnosed disease. Early and accurate diagnosis and also treatment for patient survival are required.
Case we report a 62-years-old men with diagnosis of Stanford Type A acute aortic dissection misdiagnosed as a hypertensive crisis. He was promptly referred to surgery. According to retro-information of the surgical team, the patient died 38 days after the operation from respiratory failure.
acute aortic dissection, hypertensive crisis, computed tomography, transthoracic echocardiography, transesophageal echocardiography
Aortic dissection (AD) is a relatively uncommon, though catastrophic disease which requires early and accurate diagnosis and treatment for patient survival [1]. The most common presenting symptom of any AD is the chest pain. However, the diagnosis of acute aortic dissection has many potential difficulties. The AD may mimic other more common conditions, such as acute coronary syndromes, pulmonary embolism, heart failure, stroke and acute abdominal illness, leading to a risk of misdiagnosis [2,3]. In sub-Saharan Africa the most frequent cardiovascular diseases are hypertension, cardiomyopathies and valvular heart disease [4]. The most common acute complication of hypertension is stroke. Acute coronary syndromes are still uncommon [4] and very little is known about the frequency of acute aortic syndromes. With aimed to highlights the physicians about this potentially fatal condition, we report a 62-years-old men with a diagnosis of Stanford Type A acute aortic dissection misdiagnosed as a hypertensive crisis.
A 62-year-old man with history of untreated high blood pressure (HBP) and heavy smoker was referred to our outpatient cardiologic clinic with a history of progressive dyspnoea and fatigue during two weeks. Fifteen days before, the patient suffered, at rest, from the sudden onset of intense back pain radiating to both arm and shoulders during 10 minutes, but did not radiated to the chest and no other symptoms were present. At that time, he visited a regional hospital and was observed HBP and its was interpreted as hypertensive crisis and triple antihypertensive therapy associated to aspirin was started. Due the persistent symptoms, he was referred to our hospital. At admission, the blood pressure (BP) was 150/50 mmHg in both arms and pulse was 92 bpm with characteristics of Corrigan pulse. Precordial examination showed normal first heart sound, loud second heart sound and a grade 3/6 diastolic heart murmur was present in aortic area. The ECG was normal. Laboratorial tests were normal. The D Dimer and cardiac biomarker were not available. Chest X-ray showed mild to moderate mediastinal widening. Transthoracic two-dimensional echocardiography (TTE) showed dilatation of the ascending aorta (51 mm) with an image suggestive of an intimal flapping that was observed in several views suggesting acute AD (Figure 1). Left ventricle was normal in dimensions with a good systolic function, no parietal hypertrophy or pericardial effusion was observed. Doppler analysis showed a moderate aortic regurgitation. The patient was admitted at the cardiac intensive care unit with the diagnosis of acute AD; beta-blocker was started. A computed tomography (CT) with intravenous contrast-enhanced showed AD of entire aorta, starting from the root of aorta and extending into the right common iliac artery, confirming the diagnosis of Stanford type A aortic dissection (Figure 2). Coronary arteries and the supraaortic branches were emerged from the true lumen. He was referred for surgery. According to retro-information of the surgical team the patient died 38 days after the operation from respiratory failure.
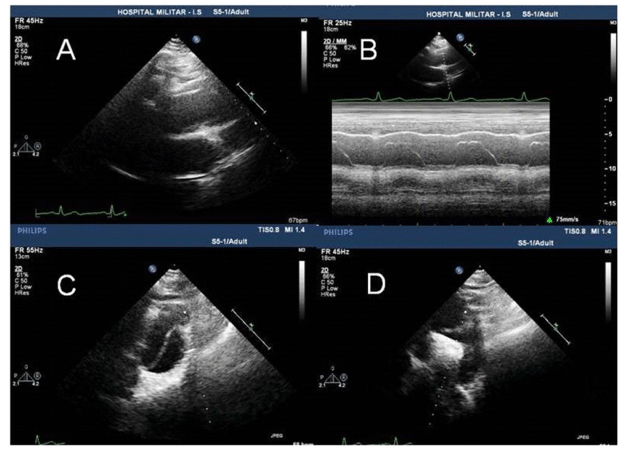
Figure 1. Doppler analysis showed a moderate aortic regurgitation.
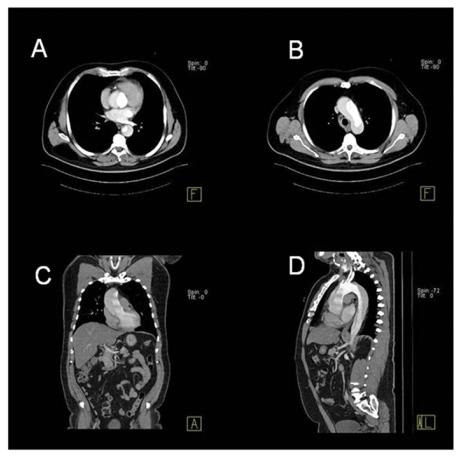
Figure 2. A computed tomography (CT) with intravenous contrast-enhanced showed AD of entire aorta, starting from the root of aorta and extending into the right common iliac artery, confirming the diagnosis of Stanford type A aortic dissection.
The incidence of acute AD in general western population is estimated to range from 2,6 to 3,5 per 100,000 person/year [5]. Twenty percent of patients with AD die before reaching the hospital and 30% die during hospital admission [6]. The Type A AD have a mortality of 1.2% per hour during the first 24-48 hours of the presentation and if left untreated up to 50% of the patients will be dead in one week [2]. Early and accurate diagnosis of AD is crucial. The consequence of dissection can include volume loss, organ ischaemia and subsequent infarction. The AD is classified as Stanford type A which involves the ascending aorta and Stanford type B which involves only the descending aorta [7]. Clinical presentation of a dissecting aneurysm is that of the sudden onset of “tearing” chest pain which radiates to the back, with associated pulse or BP deficit between the upper limbs and a abnormal chest X-ray witch is present in only one-third of patients [2]. Our patient was oligosymptomatic. The AD is a dynamic process and can occur in any part of the aorta, patients can present with a wide variety of symptoms based on where the false lumen arises, how far the dissection has spread and whether vessels arise from the true or false lumen. When the patient came to our hospital fifteen days have past from the onset of the disease and he presented symptoms of heart failure and typical signs of aortic insufficiency and associated with image findings was possible to close the diagnosis of acute AD. In fact, the typical patient in IRAD registry [2] is a male in his seventh decade with a history of hypertension, who presents with abrupt onset of chest pain [2]. The majority of patients with acute AD had abnormalities in thoracic X-ray. However 10 to 20% of the patients had a normal thoracic X-ray, so a normal thoracic X-ray cannot rule out the diagnosis. For definitive diagnosis of acute AD, imaging such as cardiac CT, magnetic resonance imaging (MRI), p (TEE) or aortography must be performed. CT is the most often used modality to diagnose of AD because of its high specificity and sensitivity and it availability. However CT had limitations since it cannot detect aortic regurgitation. MRI also has high specificity and sensitivity, but the test is time consuming and less available. TEE is usually used for hemodynamically unstable patients since it can be performed at the bedside or in an operating room for emergency. In spite of previously reported moderate specificity and sensitivity of transthoracic echocardiography (TTE) [8], in patient presented herein the TTE was used as the first imaging modality because it was promptly available, the patient had signs and symptoms of aortic regurgitation and a high probability to have an AD; the result confirmed the suspension. The TTE have been described as an useful imaging modality for the diagnosis of classic acute type A AD [9]. In order to establish definitive diagnosis, better characterize the AD and to decide the therapeutic option; we perform a CT, once the patient was hemodynamically stable. According to the guideline of American Heart Association [1] patients with risk score 2 or 3 (abrupt onset back pain, systolic BP differential, murmur of aortic insufficiency) have to undergone immediate aortic imaging evaluation and surgical consultation, as in our case. The initial management of an AD is the same independent of the type. The patient should be kept pain free and their blood pressure controlled using intravenous beta blockers. Patient who have a type A dissection, if they are fit for surgery will require emergency operative repair. Patients who have a type B dissection can be managed medically and monitored for the development of complication which will determine if surgical intervention is required. Our patient had a Stanford type A aortic dissection, so he was promptly referred for surgery. Overall, in-hospital mortality rates in patients with type A AD remains high with nearly 1/3 of patients, as in this case, even in centers that have extensive expertise and interest in the treatment of these high-risk patients [10].
In conclusion, this case highlights the importance of clinical suspicion of this potentially life condition. Despite optimal medical and surgical approach, prompt diagnoses is extremely important to life saving. Physicians must always be aware of aortic dissection in presence of HBP, symptom of the sudden onset of back/chest pain and should investigate properly other signs of aortic dissection.
- Baliga RR, Nienaber CA, Bossone E, Oh JK, Isselbacher EM, et al. (2014) The role of imaging in aortic dissection and related syndromes. JACC Cardiovasc Imaging 7: 406-424. [Crossref]
- Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, et al. (2000) The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 283: 897-903. [Crossref]
- Zhan S, Hong S, Shan-Shan L, Chen-Ling Y, Lai W, et al. (2012) Misdiagnosis of aortic dissection: experience of 361 patients. J Clin Hypertens (Greenwich) 14: 256-260. [Crossref]
- Brink AJ, Aalbers J (2009) Strategies for heart disease in sub-Saharan Africa. Heart 95: 1559-1560. [Crossref]
- Clouse WD, Hallett JW Jr, Schaff HV, Spittell PC, Rowland CM, et al. (2004) Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc 79: 176-180. [Crossref]
- Mehta RH, O'Gara PT, Bossone E, Nienaber CA, Myrmel T, et al. (2002) Acute type A aortic dissection in the elderly: clinical characteristics, management, and outcomes in the current era. J Am Coll Cardiol 40: 685-692. [Crossref]
- Braverman AC, Thompson RW, Sanchez LA (2012) Diseases of the Aorta. In Braunwald’s Heart Disease a Textbook of Cardiovascular Medicine. Ninth Edition. Elsevier Saunders 1309-37.
- Erbel R, Aboyans V, Boileau C, et al. (2014) ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 35: 2873-926.
- Cecconi M, Chirillo F, Costantini C, Iacobone G, Lopez E, et al. (2012) The role of transthoracic echocardiography in the diagnosis and management of acute type A aortic syndrome. Am Heart J 163: 112-118. [Crossref]
- Mehta RH, Bossone E, Evangelista A, O'Gara PT, Smith DE, et al. (2004) Acute type B aortic dissection in elderly patients: clinical features, outcomes, and simple risk stratification rule. Ann Thorac Surg 77: 1622-1628. [Crossref]
2021 Copyright OAT. All rights reserv


