Cervical cancer is the leading cause of cancer-related death in women world-wide. Recently, the post-translational modification, β-N-acetylglucosaminylation (O-GlcNAcylation), has been discovered in many types of epithelial cancers, including those of the female reproductive tract, but the role it potentially plays in cellular growth and metastasis is unclear. This study investigated the effect of global O-GlcNAcylation on the tumorigenic properties of the cervical cancer cell line, SiHa; including cytokeratin 8/18 (K8/18) intermediate filaments as a potential target. Overall, inhibition of O-GlcNAcylation (via the inhibitor, OSMI-1) in SiHa cells impaired cell proliferation (p<0.01) and invasion (p<0.01), yet did not affect cell cycle progression. These effects occurred concomitantly with an alteration of cellular morphology, principally the disruption/decline of K8/18 and β-actin filament expression. The results suggest O-GlcNAcylation regulates several aspects of tumorigenesis in cervical cancer cells, and cytoskeletal proteins are among the targets.
cervical cancer, O-GlcNAc, cytokeratins, tumorigenicity
Abbreviations
O-GlcNAc: β-N-acetylglucosamine; K8/18: cytokeratin 8/18; HPV: Human Papillomavirus; HR-HPV: High Risk HPV; OGT: O-GlcNAc Transferase; CHX: Cycloheximide; UDP: Uridine diphosphate; BSA: Bovine Serum Albumin; EMEM: Eagle’s Minimum Essential Medium; DMSO: Dimethyl Sulfoxide; AKT: Protein Kinase B; OSMI-1: OSMI-1; IHC: Immunohistochemistry; PI: propidium iodide
Cervical cancer is among the most common types of cancer world-wide, and is the most readily diagnosed form of cancer for women in developing countries [1]. Infection by human papillomavirus (HPV) is the primary cause of cervical cancer, with approximately 97% of all cervical cancer patients testing positive for HPV DNA [2]. Fifteen types of HPV are classified as high-risk (HR-HPV) based upon their prevalence and oncogenic nature [2], with two types (HPV 16 and 18) being closely related and contributing to ~71% of all cervical cancer tumors [3]. In less-developed nations, cervical cancer continues to be the leading cause of cancer-related death in women [3,4]. Prophylactic measures to prevent cervical cancer in these parts of the world present logistical challenges, so there is increasing urgency to gain fundamental insight about disease progression and to develop therapeutic methods that will ultimately eliminate the problem.
In many epithelial cancers, disease onset and progression are associated with increases in post-translational modification of cellular proteins, including β-N-acetylglucosaminylation (O-GlcNAcylation) [5-7]. O-GlcNAcylation is a unique form of glycosylation that results in the addition of a single sugar moiety, β-N-acetylglucosamine (GlcNAc), to proteins at serine and threonine residues. The enzyme, O-GlcNAc Transferase (OGT) adds O-GlcNAc to the proteins via the substrate UDP-GlcNAc, the end product of the hexosamine biosynthesis pathway (HBSP). Conversely, the enzyme O-GlcNAcase (OGA) removes O-GlcNAc from the targeted proteins [8]. In this manner, the reversible and dynamic nature of O-GlcNAc modification is akin to phosphorylation of cellular proteins rather than simply glycosylation. Because O-GlcNAcylation occurs on serine and threonine residues, it can dynamically influence O-phosphorylation [9]. Proteins can be reciprocally modified by O-GlcNAc and O-phosphate (e.g., estrogen receptor β [10]), while in other instances O-GlcNAc competes with phosphorylation physiologically via steric hindrance [11]. Still other proteins, for example many cytokeratin intermediate filaments, exhibit both forms of modifications, but at distant sites [12].
The most common type of cytokeratin intermediate filament expressed in simple epithelial cells is the heterodimer, cytokeratin 8/18 (K8/18). The type I monomer, cytokeratin 18 (K18), is O-GlcNAcylated at three serine residues in the head domain, while still highly-phosphorylated in the tail domain [13,14]. The major biological function of cytokeratins is structural, but K8/18 filaments are also important in many cellular functions including apoptosis, mitosis, cell cycle progression, and cell signaling [15-17]. Additionally, K18 of K8/18 filaments is widely-used as a diagnostic tool and biomarker for many epithelial cancers [15,18,19], and is known to promote metastasis [18]. Importantly, the dynamic equilibrium of K8/18 filament stability between soluble and filamentous forms is crucial in determining cellular function, and this stability is regulated by both site-specific phosphorylation and O-GlcNAcylation [21].
High intracellular concentrations of O-GlcNAc and high levels of O-GlcNAcylation are common among many cancers, yet the role of this form of glycosylation in disease progression is less understood. Additionally, there are a variety of endpoints to measure metastatic potential, but the extent to which O-GlcNAcylation impacts these measures in cervical cancer is unknown. The objective of the current study was to investigate the impact of global O-GlcNAcylation on cervical cancer cells, hypothesizing that O-GlcNAcylation augments metastatic potential, in part by influencing K8/18 filaments and cytoskeletal re-organization.
Cell culture and reagents
The human cervical cancer cell line, SiHa (ATCC® HTB35™), derived from the cervical tumor of a HR-HPV 16-infected patient was used [22]. At present, there is no information available indicating the state of O-GlcNAcylation or other forms of glycosylation within this cell line. For culture, the SiHa cells were maintained in Eagle’s Minimum Essential Medium (EMEM), supplemented with 10% fetal bovine serum (FBS) and antibiotic-antimycotic, all purchased from Thermo Fisher Scientific (Waltham, MA). The cells were incubated in a humidified, 5% CO2 environment at 37°C. Global inhibition of O-GlcNAcylation (Hypo- O-GlcNAcylation) was achieved by exposing the cells to the OGT inhibitor, OSMI-1 (50μM) as described previously by others [22,23], and was verified by immunoblotting (described below). The OSMI-1 was purchased from Aobious (Gloucester, MA) dissolved in DMSO (Thermo Fisher), and the cells were exposed for a period of 9-24 hours depending upon the experimental endpoint (described below). Additional cultures of SiHa cells were exposed to an equivalent amount of DMSO (1μl/ml) as a negative control.
Immunoblotting for global O-GlcNAcylation
Whole cell lysates of SiHa cells cultured in T-25 flasks (Corning, Corning, NY) for 48 hours, were harvested by scraping, and separated on pre-stained SDS-Page gels (BioRad, Hercules, CA) and transferred to PVDF membrane. Membranes were probed for global O-GlcNAcylation (CST-O-GlcNAc 110.6 antibody; Cell Signaling Technology, Inc.; Beverly, MA) and then secondary antibody (Anti-rabbit IgG HRP-linked Antibody, Cell Signaling Technology, Inc.), followed by Clarity Western ECL substrate (BioRad). Blots were imaged on the BioRad ChemiDoc Imaging System.
Cell proliferation assay
In a set of 3 independent experiments, cells were seeded in duplicate in flat-sided Thermo Fisher Scientific™ Nunc™ Cell Culture Tubes at an initial seeding density of 60k cells/ml of culture medium. Treatment groups included cultures of cells continuously exposed to vehicle (DMSO-Control) or 50μM OSMI-1. The conditioned culture medium was exchanged without/with treatment daily, and the cells for each group were harvested for counting at 24-hour intervals over a seven-day period.
Flow cytometric analysis of cell cycle progression
In a set of 3 independent experiments, SiHa cells were seeded at a density of 100,000 cells/ml in T-25 flasks (Corning) and treated without or with OSMI-1 in culture through the exponential growth phase before being enzymatically harvested and fixed/permeabilized with 70% ethanol. The cells were then stained with propidium iodide (PI) and fluorescence was measured with a 488nm laser (610/20 BP Filter) on a BD LSRII Flow Cytometer. Data were analyzed with Flow Jo Analysis Software.
Caspase-Glo 3/7 assay for Fas-induced apoptosis
In a set of 3 independent experiments, SiHa cells were seeded in triplicate into 96-well plates (Corning™ Costar™ 96-Well White Clear-Bottom Plates) at a density of 20,000 per well in 100 μL of complete media and then cultured without or with OSMI-1 (50 µM) for 24 hours. The cells were then pretreated for 30 min with the protein synthesis inhibitor, 30 µg/ml Cycloheximide (CHX; Sigma-Aldrich), followed by 1 µg/ml FasL antibody (CH11; EMD Millipore), or 10 µM Staurosporine (Sigma-Aldrich) for 8 hours. The incidence of apoptosis was determined using a caspase enzyme assay, following the manufacturer’s recommendations (Caspase-Glo 3/7 Assay; Promega, Madison, WI).
Cell migration invasion assay
Three independent in vitro cell migration-invasion experiments were conducted to measure metastatic potential of the SiHa cells. The assays were conducted using Corning® Transwell® polycarbonate membrane cell culture inserts for 24 well plates (VWR, Radnor, PA) containing a Tissue Culture-treated polycarbonate membrane filter (6.5 mm diameter, 8 μm pore size). This approach has been described previously by others in similar experiments [24-26]. Briefly, the upper chamber of each well was seeded with SiHa cells (50K cells/well) in serum-free medium without or with OSMI-1 (50 µM) and the lower chamber contained medium supplemented without or with 10% FBS (chemoattractant). Treatments for each experiment were conducted in triplicate. After 24 hours of incubation at 37°C and 5% CO2, the cells on the top of the membrane (non-invasive) were removed with a cotton swab, and the inserts were fixed and stained with 600 μl of crystal violet (Thermo Scientific). Invasive cells that had penetrated the membrane were photographed (20X magnification). Invasiveness was also quantified by de-staining the membranes in 600 μl of 70% ethanol for 10 minutes and then measuring the absorbance at 540 nm.
Immunocytochemistry of cytoskeletal proteins
SiHa cells were cultured in 12 well plates (100K cells/well) on glass cover slips using conditions described above for 24 hours, then fixed and permeabilized with 100% methanol. Primary antibodies for the detection of K18 in the K8/18 filaments (CY90 antibody, Cell Signaling Technology, Inc.), α-tubulin (236-10501 antibody, Thermo Fisher Scientific, Waltham, MA), and β-actin (13E5 antibody, Cell Signaling Technology, Inc.) were applied, followed by wash steps and detection using the secondary antibody (Anti-rabbit or IgG, HRP-linked Antibody, Cell Signaling Technologies) and AEC substrate kit (Vector Laboratories, Burlingame, CA). The cells were counterstained with hematoxylin (Vector), mounted onto slides, and imaged at 40X.
Statistical analysis
All experiments were independently repeated a minimum of three times, using a fresh aliquot of SiHa cells (passage 4-7) to initiate each experiment. Data were analyzed by one- or two-way analysis of variance (ANOVA), followed by a Student’s T post-test for multiple comparisons; differences among means were considered significant at p<0.05.
Hypo-O-GlcNAcylation of SiHa cells impairs cell proliferation and invasion
A variety of studies have demonstrated that the state of global O-GlcNAcylation in cells, including epithelial-derived cancer cells, can have profound physiological effects on growth and development. In the current study, the effect of global O-GlcNAcylation on proliferation, invasion, and cytoskeletal organization was assessed in a cervical cancer cell line (SiHa cells). To our knowledge, this is the first report of the effects of the highly-specific O-GlcNAc Transferase (OGT) inhibitor, OSMI-1, on cervical cancer cells. The ability of OSMI-1 to globally impair O-GlcNAcylation, without affecting SiHa cell viability, was clearly evident in these experiments, and was sustained for an extended period (Figure 1). Cell proliferation was assessed by monitoring growth curves through the death phase, and revealed that the lack of O-GlcNAcylation (Hypo-O-GlcNAc) impaired proliferation (p<0.01) over the entire length of the experiment (Figure 2A). Overall, the doubling rate for controls was more than three-fold higher per day than the Hypo-O-GlcNAcylated cultures (0.7 vs 0.2 doublings/day; p<0.01) when averaged across the experiment. These results suggest O-GlcNAcylation regulates mitosis, possibly via the cell cycle [32]. Indeed, others have reported the cell cycle is accelerated (G1àS) following exposure to the GFAT1 inhibitor, DON, which reduces global O-GlcNAcylation [28]; whereas the same study determined that increased O-GlcNAcylation delays G2/M progression, possibly through induced changes to OGT and OGA activity. These findings illuminate the complex, sometimes unpredictable, nature of O-GlcNAcylation in cells. In the current study, however, hypo-O-GlcNAcylation via OSMI-1 had no effect on parameters of cell cycle progression (Figure 2B), indicating that SiHa proliferation is impaired in some other way.
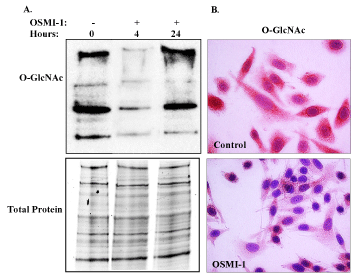
Figure 1. Inhibition of global O-GlcNAcylation by OSMI-1. [A] Immunoblot analysis of global O-GlcNAc in SiHa whole cell lysates following treatment with OSMI-1 for 4 and 24 hours. O-GlcNAcylation of cellular proteins declined within 4 hours of OSMI-1 treatment and remained diminished through 24 hours. There was no effect of OSMI-1 on total protein expression, as indicated in the lower panel [B] Impaired O-GlcNAcylation was also evident in fixed, cultured cells, where immunostaining for O-GlcNAcylation was reduced throughout the cytoplasm of OSMI-1 treated cells, 24 hours after treatment (40X magnification). Representative images of 3 independent experiments.
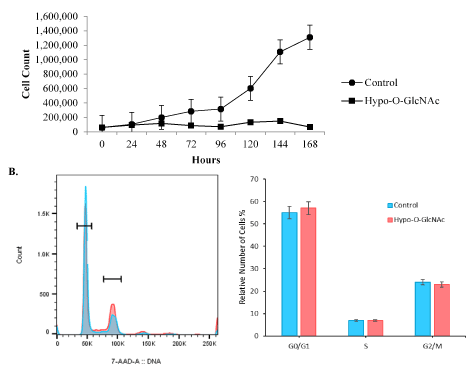
Figure 2. SiHa cell proliferation is impaired by Hypo-O-GlcNAcylation. [A] SiHa cell proliferation was impaired (p<0.01) by Hypo-O-GlcNAcylation following OSMI-1 exposure. Total number of generations, multiplication rate, and generation time for control versus Hypo-O-GlcNAcylated cells were all reduced following 3 independent experiments. [B] Flow cytometric analysis of parameters of cell cycle progression revealed no effect of Hypo-O-GlcNAcylation when comparing control and OSMI-1 treated cultures.
Observing that hypo-O-GlcNAcylation impaired cell proliferation, but not viability, we then investigated the effect of this post-translational modification on aspects of cellular metastasis by evaluating the relative invasiveness of SiHa cells. Both global and specific O-GlcNAcylation of proteins and transcription factors are linked to increased cell invasiveness/migration in breast and ovarian cancers [33-35]. Similarly, upregulation of O-GlcNAc enzymes is associated with myometrial invasive cells in endometrial cancer [36]. Our results are consistent with these findings. Control cultures of SiHa cells exhibited an aggressive phenotype, characterized by cell penetration through the Transwell® polycarbonate membrane insert within 24 hours of culture (Figure 3A). Conversely, hypo-O-GlcNAcylation reduced cell invasion (p<0.05; Figure 3B), suggesting that O-GlcNAcylation augments the mobility of the cells. Overall, the results indicate that O-GlcNAcylation, specifically through the actions of OGT, heightens the tumorigenic potential of cervical cancer cells by accelerating both proliferation and invasion capabilities. These observations agree with a recent study of OGT manipulation in colon cancer cell lines, wherein genetic silencing of OGT impaired cell invasiveness and migration [32].
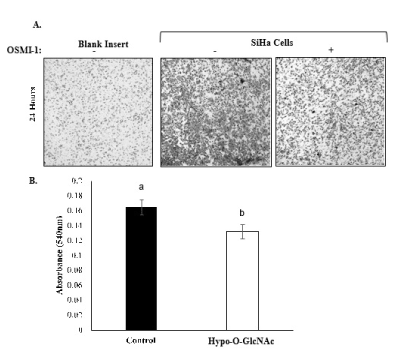
Figure 3. Effect of Hypo-O-GlcNAcylation on SiHa cell invasion. [A] Representative images (10X) depicting SiHa cells that have penetrated the Transwell inserts in response to chemoattractant. Note that OSMI-1 treatment impaired cell penetration. [B] A bar graph comparing the invasiveness of SiHa cells in control and Hypo-O-GlcNAcylated cultures for 3 independent experiments (Mean ±SEM) is shown. Bars with different letters denote differences (p<0.05).
Hypo-O-GlcNAcylation affects cytoskeletal morphology, but not Fas-induced apoptosis
Cytoskeletal proteins are fertile targets for post-translational modification, including O-GlcNAcylation, so it is plausible for O-GlcNAcylation to affect cell morphology. Indeed, SiHa cells exhibited pronounced changes in morphology following OSMI-1 treatment compared to control cells (Figure 4). Notably, immunostaining for K18 and β-actin proteins was diminished in Hypo-O-GlcNAcylated cells, and the cells generally had a flattened, stellate morphology compared to the more spindle-like appearance of control cells (Figure 4). For K18, in particular, staining was uniformly detectable throughout the cytoplasm in control cells; whereas it remained primarily perinuclear, and somewhat aggregated as it extended to the periphery in Hypo-O-GlcNAcylated cells (Figure 4). Similar forms of filament reorganization have been noted in other epithelial cancers [39], and are believed to be a direct result of K8 phosphorylation in K8/18 filaments [40]. In the current study, there was no evidence that α-tubulin expression was affected by hypo-O-GlycNAcylation (Figure 4), despite the observation that K18 and β-actin expression were both diminished. Others have observed that α-tubulin and β-actin expression in colon cancer cells are unaffected by inhibition of OGT, yet the morphology of these cells is “stocky and stunted” [31]. Although K8/18 filament expression was not evaluated, the results of the previous study did support the concept that hypo-O-GlcNAcylation disrupts cytoskeletal organization. Beyond a structural role, K8/18 filaments also influence cell survival, specifically providing resistance to Fas-induced apoptosis [14,17,37]. In the present study, we found that SiHa cells are indeed resistant to Fas-induced apoptosis, but such resistance is not influenced by hypo-O-GlcNAcylation (Figure 5; p>0.05), or associated with a loss of K8/18 filament expression (Figure 4). This suggests the mechanism(s) of Fas resistance in SiHa cells involves processes other than O-GlcNAcylation and proteins other than K8/18 filaments. Additionally, as noted above, the overall viability of the SiHa cells remained unaffected by hypo-O-GlyNAcylation, as measured by caspase 3/7 enzyme activity (Figure 5; Control vs. Hypo-O-GlycNAcylated, p>0.05). Thus while other investigations have concluded O-GlcNAcylation is fundamental to cell viability, especially in embryonic stem cells [31], the extent of impairment of O-GlcNAcylation in the current study was insufficient to hinder cell survival.
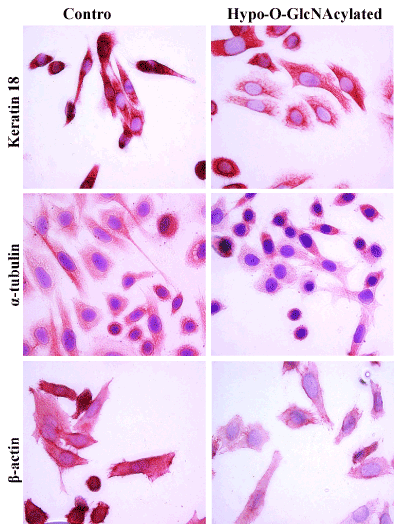
Figure 4. Effects of Hypo-O-GlcNAcylation on SiHa cell morphology and cytoskeletal expression. Representative images (40X) of immunocytochemical staining for keratin 18, α-tubulin, and β-actin in SiHa cells are shown. Hypo-O-GlcNAcylation reduced both Keratin 18 and β-actin expression. There were no obvious alterations of α-tubulin expression observed. These outcomes were evaluated following three independent experiments.
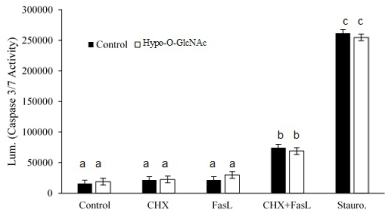
Figure 5: Effect of Hypo-O-GlcNAcylation on SiHa cell sensitivity to apoptosis. A bar graph depicts the relative sensitivity of SiHa cells (Mean ± SEM) to cytokine- (FasL) and chemotherapeutic- (Staurosporine) induced apoptosis (as measured by cleaved caspase 3/7 activity) following three independent experiments. Cytokine in the presence of the protein synthesis inhibitor, cycloheximide (CHX), induced apoptosis (p<0.05), but impairment of O-GlcNAcylation did alter the sensitivity of the cells to FasL. Staurosporine was used as the positive control for the assay. Bars with different letters denote differences (p<0.05).
Overall, O-GlcNAcylation enhances the tumorigenic properties of SiHa cells by accelerating cell proliferation, and augmenting invasive and migratory capabilities. Conversely, hypo-O-GlcNAcylation alters cellular morphology and cytoskeletal organization, in part by reducing the expression of K8/18 and β-actin filament proteins. The results support the hypothesis that O-GlcNAcylation augments metastatic potential of cervical cancer cells, and suggest there is a physiologic connection between O-GlcNAcylation and cytoskeletal reorganization, ostensibly involving the modulation of O-phosphorylation of K8/18 filaments, but this warrants further study.
NMJ, CH, and SP performed the experiments, conducted the statistical analyses, and assisted in the preparation of the manuscript. NMJ and DHT conceived the study, participated in its design and coordination, and wrote the manuscript. The flow cytometry data we presented were obtained at the Harry Hood Bassett Flow Cytometry and Cell Sorting Facility, University of Vermont College of Medicine. This material is based upon work supported by the National Science Foundation Graduate Research Fellowship under Grant No. DGE 1450271 (NMJ) and by the UNH COLSA Karabelas Fund (DHT).





