Abstract
Cytomegalovirus (CMV) is one of eight herpes virus that can cause disease in humans affecting the 30-100% of the population. In the oral cavity, CMV infection appears as a common ulcerated lesion, without clinical pathognomonic signs. The locations of oral lesions are hard palate, soft palate, tongue and floor of the mouth. This case report describes the case of a 59-year-old woman with a painful roundish ulcerated lesion covered with fibrinous exudate located on the cheek. The patient underwent kidney transplantation for a reduced kidney function secondary to the Good pasturesyndrome and was receiving immunosuppressive chemotherapy. The ulcer improved rapidly following the initiation of ganciclovir treatment.
- Introduction
Cytomegalovirus (CMV) is one of eight herpes virus that can cause disease in humans. It can affect the 30-100% of the population [1]. Cytomegalovirus is a double-stranded DNA virus with a cap-sid protein and lipoprotein envelope [2]. Its replication leads to the formation of large nuclear inclusions and smaller cytoplasmic inclusions. CMV has a strong propensity to develop disease and usually affects patients with congenital immune deficiency, acquired or iatrogenic, and AIDS patients at an advanced stage, transplanted patients, patients on immunosuppressive chemotherapy, infants with infected mothers [3].
In the case of transplanted patients, events associated to CMV do not show differences related to sex, patient age at transplantation time, cause of transplantation, type of immunosuppressive chemotherapy in progress [4].
CMV is characterized by tropism for T-lymphocytes and has a slow replication cycle and the ability to persist in a latent form after primary infection in cells of the reticuloendothelial system, in salivary cells and kidney cells [5].
CMV can affect through saliva, sexual contact, blood transfusion, organ transplantation or hematopoietic stem cell line. Placental infection occurs more commonly during primary infection and symptomatic congenital infection is more likely to occur in babies of women without pre-existing immunity to CMV. CMV may be present in both cervical secretions and in breast milk. Most children infected in utero appear healthy but may manifest late sequelae. Those children born with cytomegalic inclusion disease have a poor prognosis. In about 10-20% of cases, follow-up shows neurological damage [6].
In the oral cavity, CMV infection appears as a common ulcer, without clinical pathognomonic signs.
The oral location can be the hard palate, soft palate, tongue and floor of the mouth [7].
Clinical case
A 59-year-old woman referred to our observation with complaints of a painful roundish ulcer, 1.2 x 1 cm, covered by fibrinous exudate, lasting for 1 month (Figure 1). The patient did not remember trauma or other possible causal events. The patient reported kidney transplantation for a reduced renal function secondary to Goodpasture syndrome. She was still on immunosuppressive therapy.
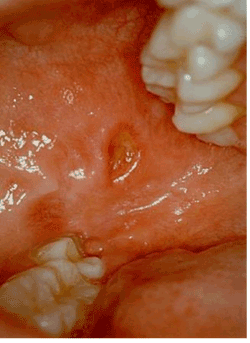
Figure 1:The painful roundish ulcerated lesion covered by fibrinous exudate located on the cheek (1.2 x 1 cm).
On oral examination, it is showed a painful ulcer with clinical signs of an inflammatory process, such as hyperemia, and edema, moreover fibrinous membrane was covering the lesion. With the aim of a correct diagnosis, an incisional biopsy was performed on the lesion area. After staining with haematoxylineosin, the microscopic examination showed the presence of cytomegalic cells, a sign of viral infection (Figure 2). Cytomegalic cells were 2 or 4 times (25-35 µ) larger than the surrounding cells and contained basophilic nuclear inclusions with a diameter of 8-10 µ, decentralized and surrounded by a clear halo giving them a typical appearance of "owl eye" (Figure 3). In addition, the nuclear membrane appeared thickened and there were frequently small granular cytoplasmic inclusions in epithelial, endothelial and stromal cells. The immunohistochemical coloration with monoclonal "anti-CMV" antibodies to detect viral proteins was positive. The ulcer improved rapidly following the initiation of ganciclovir treatment.
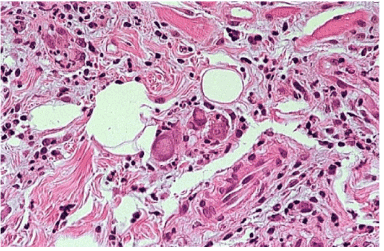
Figure 2:In the bioptic samples taken from the lesion site stained with haematoxylin-eosin; we found cytomegalic cells, 2 or 4 times (25-35 µ) larger than the surrounding cells and containing basophilic intranuclear inclusions with a diameter of 8-10 µ.
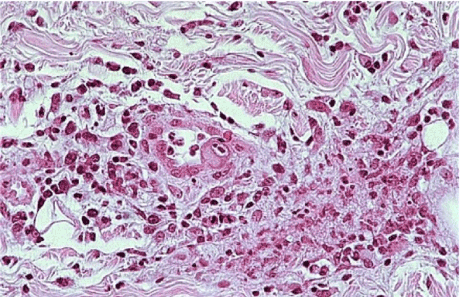
Figure 3:Besides, a thickening of nuclear membranes was present and small granular cytoplasmic in clusions in epithelial, endothelial and stromal cells were frequent in the sample.
Discussion
The oral ulcers are among the most common disorders of the oral mucosa. The patient may come to the dentist observation with the unique manifestation of the pain symptom caused by the ulcer it-self.
Several factors can determine oral ulcers or they can be local manifestations of various systemic diseases.
Most of oral ulcers are caused by physical or chemical trauma [8], in particular connected with the recruitment of inappropriate drugs, such as salicylic acid and its derivatives or the use of drugs, that can cause burns of the mucous and oral ulcers [9]. Oral ulcers may occur during Coeliac disease [10] or Crohn disease [11], a chronic disease that can involve the gastrointestinal tract including the oral mucosa; in 9% of patients oral ulcers can be the first clinical manifestation [12]. The ulcerative colitis can determine oral ulcers as possible manifestations [13]. Several authors reported the presence of oral lesions in patients with the gastro-intestinal tract diseases with high frequency [14].
Oral ulcers can be present in lichen planus [15], syphilis [16,17], tuberculosis [18], lymphoma [19-21], and may be a sign of oral cancer [22]. The recurrent aphthous stomatitis is possible in presence of oral ulcers [23]. Viral infections, particularly HSV1, HSV2, and, more rarely, EBV and HIV; in presence of a state of immunodeficiency, may induce some superficial oral ulcers, often associated with labial manifestations [24-27]. Bacterial infections are associated with particular manifestations such as necrotizing gum [28]. Other infective agents can cause an oral ulcer, such as candida or other mycetes [29-33].
2021 Copyright OAT. All rights reserv
Ulcers, according to the different pathogenesis, can have a characteristic localization such as hard palate and soft palate in ulcerative colitiss [12], or a gingival location in the gingivitis necrotizing infections caused by bacteria, poorly controlled diabetes mellitus, immunodeficiency, poor oral hygiene, measles and malnutrition [12,34]. Sometimes, however, the clinical appearance does not allow performing a diagnostic hypothesis.
Histology and immunohistochemistry examinations allow definitive diagnosis, revealing if the ulcers are the oral manifestation of a systemic disease or a single clinical lesion [35,36].
Oral ulcer can be attributed to CMV in patients with AIDS, in patients receiving immunosuppressive chemotherapy, in patients with heart or bone marrow transplantation [37-41].
Opportunistic infections are common in immunosuppressed patients after renal transplantation. CMV infection occurs in 44%–85% of kidney transplant patients [4]. CMV infection in renal transplant recipients exhibits a wide range of clinical manifestations; it can affect any segment of the gastrointestinal tract [4]. However intra-oral localization is rare [3,4]. López-Pintor et al. reported 6 cases of oral ulcerations due to CMV in renal transplant recipients in 2009 [3].
Clinical diagnosis of oral ulcers due to CMV in immunosuppressed patients can be difficult because various etiologic factors, such as infections, drugs, and tumors may produce similar lesions [3]. Often the diagnosis can be confirmed only by resolution of ulcers with ganciclovir therapy [24]. The early diagnosis of oral lesions due to CMV is important because CMV infection causes infectious disease syndromes, increases immunosuppression, and is associated with opportunistic infections and allograft rejection episodes in renal transplant recipients [3].
The case treated underlines the importance to perform a complete collection of anamnestic data. The clinical history of the patient allowed the identification of this oral ulcer pathogenesis. The subsequent histological exam confirmed the initial diagnostic hypothesis and allowed to set a fast and effective drug therapy.
Conclusion
Considering the wide variety of pathological conditions that can lead to the onset of oral ulcers and their role in the patient health, it is evident the importance of a correct diagnosis for the appropriate treatment of this lesion, particularly in relation to the choice of appropriate therapy in a patient with a complex medical history. CMV oral ulcers represent a possible manifestation in immunodeficient patients. It may be difficult for clinicians to distinguish between CMV lesions and ulcers from other disease. To obtain a careful and correct diagnosis clinicians must perform histological and immunohistochemical analysis, that revealed the presence of CMV infection.
References
- Jain M, Duggal S, Chugh TD (2011) Cytomegalovirus infection in non-immunosuppressed critically ill patients. J Infect Dev Ctries 5: 571-579. [Crossref]
- Mcgavran MH, Smith MG (1965) Ultrastructural, cytochemical, and microchemical observations on cytomegalovirus (salivary gland virus) infection of human cells in tissue culture. Exp Mol Pathol 76: 1-10. [Crossref]
- López-Pintor RM, Hernández G, de Arriba L, Morales JM, Jiménez C, et al. (2009) Oral ulcers during the course of cytomegalovirus infection in renal transplant recipients. Transplant Proc 41: 2419-2421. [Crossref]
- Ponticelli C, Passerini P (2005) Gastrointestinal complications in renal transplant recipients. Transpl Int 18: 643-650. [Crossref]
- Gandhi MK, Khanna R (2004) Human cytomegalovirus: clinical aspects, immune regulation, and emerging treatments. Lancet Infect Dis 4: 725-738. [Crossref]
- Buonsenso D, Serranti D, Gargiullo L, Ceccarelli M, Ranno O, et al. (2012) Congenital cytomegalovirus infection: current strategies and future perspectives. Eur Rev Med Pharmacol Sci 16: 919-935. [Crossref]
- Ueda T, Ogata H, Kojima Y, Ishida E (2014) Cytomegalovirus oral ulcers. Infection 42: 235.
- Thompson LD (2011) Oral traumatic ulcer. Ear Nose Throat J 90: 518-534. [Crossref]
- Gandara-Rey JM, Diniz-Freitas M, Gandara-Vila P, Blanco-Carrion A, Garcia-Garcia A (2002) Lesions of the oral mucosa in cocaine users who apply the drug topically. Med Oral 7: 103-107. [Crossref]
- Campisi G, Di Liberto C, Carroccio A, Compilato D, Iacono G, et al. (2008) Coeliac disease: oral ulcer prevalence, assessment of risk and association with gluten-free diet in children. Dig Liver Dis 40: 104-107. [Crossref]
- Brennum J, Hjelt K (1986) Crohn disease diagnosed by oral biopsy from an aphthous ulcer. Nord Med 101: 131. [Crossref]
- Leão JC, Gomes VB, Porter S (2007) Ulcerative lesions of the mouth: an update for the general medical practitioner. Clinics (Sao Paulo) 62: 769-780. [Crossref]
- Katsanos KH, Torres J, Roda G, Brygo A, Delaporte E, et al. (2015) Review article: non-malignant oral manifestations in inflammatory bowel diseases. Aliment Pharmacol Ther 42: 40-60. [Crossref]
- Harty S, Fleming P, Rowland M (2005) A prospective study of the oral manifestations of Crohn's disease. Clin Gastroenterol Hepatol 3: 886-89. [Crossref]
- Scully C, Beyli M, Ferreiro MC, Ficarra G, Gill Y, et al. (1998) Update on oral lichen planus: etiopathogenesis and management. Crit Rev Oral Biol Med 9: 86-122. [Crossref]
- Vera-Kellet C, Harz-Fresno I, Manriquez J (2014) Labial ulcer: oral manifestation of syphilis. Braz J Infect Dis 18: 570-571. [Crossref]
- Murrell GL (2009) Secondary syphilis oral ulcer. Otolaryngol Head Neck Surg 140: 942-943. [Crossref]
- Aoun N, El-Hajj G, E Toum S (2015) Oral ulcer: an uncommon site in primary tuberculosis. Aust Dent J 60: 119-122. [Crossref]
- Shapiro N (2015) Diffuse large B-cell lymphoma of the gingiva in a patient on long-term use of methotrexate being treated for psoriasis. Compend Contin Educ Dent 36: 426-428. [Crossref]
- Souto GR, Pereira TS, Castro AF, Mesquita RA (2013) Diffuse large B-cell lymphoma, not otherwise specified of the palate: A case report. J Clin Exp Dent 5: e287-290. [Crossref]
- Meng W, Zhou Y, Zhang H, Jiang L, Wang Z, et al. (2010) Nasal-type NK/T-cell lymphoma with palatal ulcer as the earliest clinical manifestation: a case report with literature review. Pathol Oncol Res 16: 133-137. [Crossref]
- Gonçales ES, Rubira-Bullen IF, Rubira CM, Miyazawa M, Chinellato LE, et al. (2007) Eosinophilic ulcer of the oral mucosa versus squamous cell carcinoma. Quintessence Int 38: 677-680. [Crossref]
- Porter SR, Scully Cbe C (2007) Aphthous ulcers (recurrent). BMJ Clin Evid [Crossref]
- Firth NA, Rich AM, Reade PC (1994) Oral mucosal ulceration due to cytomegalovirus associated with human immunodeficiency virus infection. Case report and brief review. Aust Dent J 39: 273-275. [Crossref]
- Syrjanen S, Leimola-Virtanen R, Schmidt-Westhausen A, Reichart PA (1996) Oral ulcers in AIDS patients frequently associated with cytomegalovirus (CMV) and Epstein-Barr virus (EBV) infections. J Oral Pathol Med 28: 204-209, 1999. [Crossref]
- Bunn B, van Heerden W (2015) EBV-positive mucocutaneous ulcer of the oral cavity associated with HIV/AIDS. Oral Surg Oral Med Oral Pathol Oral Radiol. [Crossref]
- Attard AA, Praveen P, Dunn PJ, James GJ (2012) Epstein-Barr virus-positive mucocutaneous ulcer of the oral cavity: the importance of having a detailed clinical history to reach a correct diagnosis. Oral Surg Oral Med Oral Pathol Oral Radiol 114: e37-39. [Crossref]
- Emslie RD (1967) Treatment of acute ulcerative gingivitis. A clinical trial using chewing gums containing metronidazole or penicillin. Br Dent J 122: 307-308. [Crossref]
- Verma RK, Shankar A, Kumar Arora S, Sivaprakash MR, Panda NK (2011) Histoplasma rhinosinusitis presenting as a nonhealing oral ulcer in an immunocompetent patient. J Otolaryngol Head Neck Surg 40: E47-50. [Crossref]
- Terai H, Shimahara M (2010) Chronic oral ulcer associated with Candida. Mycoses 53: 168-172. [Crossref]
- Vijayan C, Suprakasan S, Kumar GN, Nair PS, Jayapalan S (2007) Primary mucocutaneous histoplasmosis presents as oral ulcer. Indian J Dermatol Venereol Leprol 73: 209. [Crossref]
- Balasaraswathy P, Theerthanath (2003) Cutaneous blastomycosis presenting as non-healing ulcer and responding to oral ketoconazole. Dermatol Online J 9: 19. [Crossref]
- Verma A, Stock W, Lait M, Ferrer K, Quinn J, et al. (2002) Actinomycosis presenting as an oral ulcer in a neutropenic patient. South Med J 95: 1105. [Crossref]
- Kaplan D (1981) Acute necrotizing ulcerative tonsillitis and gingivitis (Vincent's infections). Ann Emerg Med 10: 593-595. [Crossref]
- Flaitz CM, Nichols CM and Hicks MJ (1996) Herpesviridae-associated persistent mucocutaneous ulcers in acquired immunodeficiency syndrome. A clinicopathologic study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 81: 433-44. [Crossref]
- Leimola-Virtanen R, Happonen RP, Syrjanen S (1995) Cytomegalovirus (CMV) and Helico-bacter pylori (HP) found in oral mucosal ulcers. J Oral Pathol Med 24: 14-17. [Crossref]
- Epstein JB, Sherlock CH, Wolber RA (1993) Oral manifestations of cytomegalovirus infection. Oral Surg Oral Med Oral Pathol 75: 443-451. [Crossref]
- Schubert MM, Epstein JB, Lloid ME, Cooney E (1993) Oral infections due to cytomegalovirus in immunocompromised patients. J Oral Pathol Med 22: 268-273. [Crossref]
- Ammatuna P, Campisi G, Giovannelli L, Giambelluca D, Alaimo C, et al. (2001) Presence of Epstein-Barr virus, cytomegalovirus and human papillomavirus in normal oral mucosa of HIV-infected and renal transplant patients. Oral Dis 7: 34-40. [Crossref]
- Jones AC, Freedman PD, Phelan JA, Baughman RA, Kerpel SM (1993) Cytomegalovirus infections of the oral cavity. A report of six cases and review of the literature. Oral Surg Oral Med Oral Pathol 75: 76-85. [Crossref]
- Correia-Silva Jde F, Victória JM, Guimarães AL, Salomão UE, de Abreu MH, et al. (2007) Cytomegalovirus shedding in the oral cavity of allogeneic haematopoietic stem cell transplant patients. Oral Dis 13: 163-169. [Crossref]



