Abstract
Purpose: We present the clinical and radiologic features of patients with angiolymphoid hyperplasia with eosinophilia (ALHE) of the orbit and ocular adnexa.
Methods: Data were collected on patient demographics, clinicopathological correlation and the radiologic findings of all cases with the confirmed tissue diagnosis of ALHE presenting to King Khaled Eye Specialist Hospital (KKESH), Saudi Arabia and School of Medicine of Ribeirão Preto, Brazil from 2000 to 2015.
Results: Seven cases were included with a mean age of 42 years (range, 13 to 71 years). The female-to-male ratio was 5:2. Location of the orbital lesion was temporal superior in all cases, with lacrimal gland involvement in 3 cases and bilateral involvement in 1 case. The common presenting symptoms were proptosis, dystopia and ptosis. Almost all cases had diffuse lesions, infiltrating the extra ocular muscles and adjacent fat and 1 case had a discrete mass. No cases had clinical signs of Kimura’s disease and none developed systemic lymphoma. Treatment with excision and steroid therapy resulted in good resolution in all but one case.
Conclusion: ALHE is a rare benign condition affecting middle-aged females with no relation to lymphoma. It has a distinct histopathological appearance and non-specific radiologic features. Multimodal treatment with complete surgical excision, adjunctive cytotoxic agents and steroids are usually sufficient for complete resolution of ALHE of the orbit and ocular adnexa.
Key words
angiolymphoid hyperplasia, kimura, eosinophilia, lymphocytes
Introduction
Angiolymphoid hyperplasia with eosinophilia (ALHE) is a benign slow-growing lesion most commonly affecting middle-aged women, typically occurring in the subcutaneous tissue of the head and neck and rarely involving the orbit and adnexa [1,2]. Only sporadic cases of periocular-orbital ALHE have been reported in the literature.
The term ALHE was first introduced by Wells and Whimster in 1969; subsequently Rosai et al. (1979) proposed the term histiocytoid hemangioma as a unifying concept embracing several previously described entities of skin, soft tissue, large vessels, bone, and heart [2]. A few years later, Enzinger and Weiss (1983) coined the term epithelioid hemangioma to describe an unusual benign lesion of endothelial cell proliferation. Other terms used for ALHE include pseudo-pyogenic granuloma, atypical pyogenic granuloma, inflammatory angiomatous nodule, subcutaneous angioblastic lymphoid hyperplasia with eosinophilia, intravenous atypical vascular proliferation, nodular angioblastic hyperplasia with eosinophilia and lymphofolliculosis [1].
The diagnosis of ALHE depends on histopathological evaluation. Characteristics of the lesion include vascular hyperplasia with plump endothelial cells and combined lymphocytic and eosinophilic cellular infiltrates [3].
ALHE has some similarities to Kimura’s disease and they were previously considered a different spectrum of the same condition. Both entities share clinical and histopathological features and can affect the orbit and the adnexa. However, current opinion holds that both are likely distinct clinical entities, yet the two designations are still often confused or used synonymously [4-6]. This case series describes the clinical and radiologic features and treatment of cases of ALHE with ocular involvement. To the best of our knowledge, this is the largest case series of ALHE involving the orbit and ocular adnexa.
Methods
A retrospective chart review was performed for cases of ALHE with involvement of the orbit and ocular adnexa presenting to King Khaled Eye Specialist Hospital (KKESH), Riyadh, Saudi Arabia and School of Medicine of Ribeirão Preto, Brazil from 2000 to 2015. The cases were included based on a confirmed tissue diagnosis at the respective institutions. Two pathologists reviewed the slides for confirmation of the diagnosis. Data were collected on the demographics, clinical manifestations, findings of the clinical examination, treatment and follow-up. A radiologist also reviewed all the computed tomography (CT), magnetic resonance imaging studies (MRI) images from both institutions and the diffusion-weighted images for all cases at KKESH. This study adhered to the tenets of the Declaration of Helsinki.
Results
Seven cases with the tissue diagnosis of ALHE were included in this study. There were 5 patients from KKESH and 2 from the School of Medicine of Ribeirao Preto, assisted during 5 year period. The female-to-male ratio was 5:2 and the mean age was 42 years (range, 13 to 71 years). The data for all cases are summarized in Table 1.
|
Case 1 |
Case 2 |
Case 3 |
Case 4 |
Case 5 |
Case 6 |
Case 7 |
Sex |
Female |
Female |
Female |
Female |
Female |
Male |
Male |
Age |
53 |
46 |
13 |
46 |
30 |
71 |
35 |
Nationality |
Saudi |
Saudi |
Saudi |
Saudi |
Saudi |
Brazilian |
Brazilian |
Symptoms starting |
8 years ago OD |
3 weeks |
3 weeks |
3 months |
2 years |
Not Recorded |
Not Recorded |
Laterality |
OU |
OS |
OD |
OS |
OS |
OS |
OD |
Systemic |
No asthma
No trauma |
No asthma
No trauma |
No asthma
No trauma |
No asthma
No trauma |
No asthma
No trauma |
No asthma
No trauma |
No asthma
No trauma |
Symptoms |
Diplopia
No fever |
Diplopia
No fever |
Dystopia
No fever |
Fluctuating swelling
Ptosis
No fever |
Swelling
ptosis
dystopia
Diplopia
No fever |
Intense conjunctival hyperemia and chemosis, white area (suppuration?)
Fever |
No fever |
EOM affected |
Yes |
No |
Yes |
Yes |
Yes |
Yes |
Yes |
Location |
Orbit: Roof, lacrimal gland |
Upper lid |
Orbit: Supero temporal |
Orbit: roof +Upper lid |
Orbit: Supero temporal |
Orbit: roof |
Orbit: Supero-nasal |
External exam |
Ptosis |
Palpable superolateral mass, non-tender, ptosis, chemosis, |
Upper lid swelling, palpable mass, ptosis |
Palpable well-defined soft lesion below the superior left orbital rim. No inflammation |
Mass in the superior lateral fornix |
Upper lid mass |
Not Recorded |
Visual acuity |
OD=20/20
OS=20/20 |
OD=20/30 OS=20/60 |
OD=20/20
OS=20/20 |
OD=20/20
OS=20/20 |
OD=20/20
OS=20/20 |
OD=20/30
OS = HM |
Not Recorded |
Proptosis |
Yes |
Yes |
Yes |
No |
Yes |
Yes |
Yes |
EOM |
Limitation in supra-duction OD |
Not Recorded |
Moderate upward gaze restriction OD |
Normal |
Normal |
Limited ocular motility in all directions |
Not Recorded |
Lymphadenopathy |
Parotid lymph node enlargement |
No |
No |
Sinusitis predominantly ethmoidal with old inspissated secretion in the right frontal sinus |
No |
No |
No |
Laboratory |
Normal
No Eosinophilia |
Normal
No Eosinophilia |
Normal
No Eosinophilia |
Normal
No Eosinophils |
Normal
No Eosinophils |
Lymphocytosis |
Normal
No Eosinophils |
CT scan |
Mass on orbital roof and lacrimal gland enlarged OU. Swelling of MOE OU. Increased density of the retro-orbital fat OD |
Left solid pre-septal mass. |
Supero-temporal left orbit mass. Displacement of the globe medially, inferiorly and anteriorly |
Soft tissue signal intensity ill-defined mass with ill-defined margin, predominantly in the eyelid and slightly bulky in continuity with the palpebral lobe of lacrimal gland and encircling the globe partially, separate from the left lateral rectus muscle. |
Well-defined homogenous 2.25 cm lesion in anterior posterior dimension located anterior and superior of the left orbit enhancing enlarged left lacrimal gland, scalloping of the adjacent bone. Lateral rectus muscle tendon not clearly identified and appears inseparable from the mass. |
Diffuse mass localized in the left roof of the orbit |
Diffuse mass in the antero- supero nasal right orbit |
MR |
Infiltrative mass involving all extraocular muscle and lacrimal glands |
Not Recorded |
Enhancing Supero-orbital mass preseptal mass involving the SR adjacent fat and lacrimal gland. |
A focal sizable mass with intermediate signal intensity in all pulse sequences and homogeneous contrast enhancement |
Enlarged left lacrimal gland affecting both compartments, with iso-signal in T1 images; marked low signal in T2 Peripheral/rim enhancement on post-contrast |
Not Recorded |
Not Recorded |
Biopsy |
Significant lymphocytic infiltrate in the lacrimal gland with no lymphoid follicles. Presence of histiocytic-like appearing proliferating endothelial cells |
Significant eosinophils and thick walled proliferating blood vessels |
Vascular hyperplasia with plump epithelioid endothelial cells and surrounding mixed inflammatory infiltrate including lymphocytes, histiocytes and eosinophils |
Compatible with AHLE |
Blood vessels with plump endothelial cells and similar inflammatory infiltrate with significant eosinophilia |
Large number of blood vessels with plump epithelioid endothelial cells and diffuse lymphoid infiltrate with significant eosinophilia. No fibrosis or lymphoid follicles |
Compatible with AHLE |
Treatment |
EB |
EB |
EB |
EB |
EB+IS |
SS+R |
EB+SS |
Evolution |
Good |
Chronic disease developing orbital fibrosis and lymph node involvement, |
Good |
Good |
Good |
Death |
Good |
Follow-up |
2 years |
10 years |
1 year |
3 years |
1 year |
|
3 year |
Recurrence |
No |
No |
No |
No |
No |
|
No |
OD; right eye, OS; left eye. HM; Hand Motion. EB; Excisional biopsy, IB; Incisional Biopsy, IS; Intralesional Steroids, R; Radiotherapy, E; Exenteration, S; Systemic Steroids, EOM: Extra ocular motility.
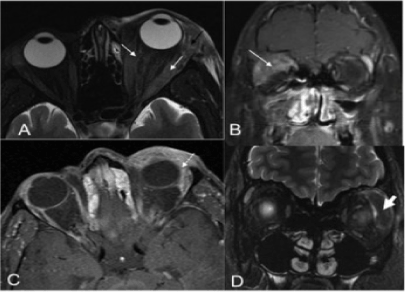
The lesion was located in the temporal superior aspect of the orbit in 6 cases and nasally in 1 case. The lacrimal gland was involved in 3 cases. The left side was involved in 5 cases and 1 patient had bilateral involvement. Proptosis was observed in 4 cases, and dystopia and ptosis in 3 cases. Restriction of ocular motility was linked to proptosis and dystopia. Vision was affected in 1 patient (case 6) who developed severe proptosis and optic nerve compression. All cases were negative for systemic lymphoma.
On the CT scan and MRI, the orbital lesions presented as diffuse masses, infiltrating the extra ocular muscles and adjacent fat with the exception of case 2 in whom the lesion was located in the pre-septal region, as a mass with a well-defined contour.
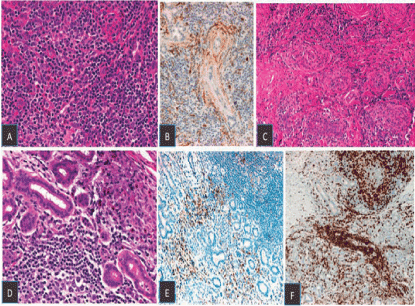
Biopsy of all cases showed the typical benign histopathological characteristics of ALHE with evidence of vascular hyperplasia and plump epithelioid endothelial cells, mixed inflammatory infiltrate with presence of eosinophils.
The lesion was removed en toto in 6 cases. Two patients received steroids, one by local infiltration of the lesion and the other systemically. One patient developed chronic inflammation and fibrosis (case 2) and another patient (case 6) expired (Table 1). After treatment there was no recurrence in all cases over a follow-up period that ranged from 1 year to 10 years.
Discussion
ALHE is a rare condition that is very unusual in the orbit and adnexa. Our case series was comprised of 5 females (Saudi) and 2 males (Brazilian). A female predominance in ALHE has been suggested for non-Oriental /non-Asian patients [7]. However, in an Asian population, ALHE occurs predominantly in males, affecting the lacrimal gland [7]. Kimura’s disease shares common histopathological features with ALHE and affects more Asians. We observed 5 cases of Saudi descent and 2, were Brazilian.
Although the majority of ALHE patients are in their 4th and 5th decades of life (Sheren et al. 1989), we have observed a wide range of age from 13 years to 71 years. The mean age in our case series was 42 years, similar to other reports [8].
Proptosis, swelling, dystopia, ptosis and lacrimal gland enlargement were the most common clinical features observed in our patients. The lesions can be asymptomatic, pruritic, painful, or pulsatile [9,10]. One patient (case 2) had reduced vision and one patient (case 6) reported loss of vision likely due to severe proptosis and optic nerve compression. Lymphadenopathy and peripheral blood eosinophilia and bronchial asthma are features of Kimura disease and not related to ALHE [8,11]. There were no cases with asthma, eosinophilia or fever in our study. However, case 1 had regional parotid lymphadenopathy.
Orbital ALHE is exceedingly rare. Notably, 6 of the 7 cases in the current case series had ALHE with orbital involvement, mainly in the upper and temporal orbit and also affecting the lacrimal gland. Sanches-Acosta et al. reported one case affecting the lacrimal gland in a female [12]. The disease in general is unilateral but one of our cases was bilateral. Shields et al. reported one case affecting both orbits with proptosis, but a sequence of biopsies excluded ALHE and confirmed the diagnosis of Kimura [13]. Barakova et al. described another bilateral case of ALHE [14].
The duration of the disease is generally shorter (1-4 years) and our patients did not have long-term disease, ranging from 3 weeks to 8 years.
ALHE with ocular adnexa and orbital involvement had nonspecific presenting signs, symptoms, and radiologic features. However, imaging studies including CT and MRI can be useful for suggesting a specific diagnosis and also to delineate the extent of the disease. The most common presenting radiological feature in our cases was a lacrimal gland mass or a diffuse infiltrative mass continuously to the subcutaneous tissue. Only case 2 was an upper lid preseptal mass. All lesions had heterogeneous high intensities on T2 weighted images except case 5, who had markedly low signal intensity on T2 weighted images. On T1 weighted images, all lesions showed slightly high- or iso-intensity for muscle. On DW images, all lesions had moderately high intensities. The mean values and standard deviation of the ADC values of the lesions and lymphadenopathies were 1.51 ± 0.33 × 10−3 mm2/s. Although most of our patients (6 of 7 cases) presented ill-defined borders on CT and MRI, Sanchez-Orgaz et al. [2] described one case of ALHE with a well-defined mass in the superior orbit and either well-defined nodular masses or ill-defined plaque like infiltrative masses in the subcutaneous tissue associated with lymphadenopathy as typical findings. Compared with that in the adjacent muscle, the signal intensity of the lesions was iso- to slightly hyper-intense on T1 weighted images and hyper-intense on T2 weighted images. The degree of enhancement of the lesions was generally greater on post-contrast MRI than on post-contrast CT scans, reflecting the higher sensitivity of MRI for detecting changes after contrast enhancement. The attenuation of the masses varies from iso- to hyper-attenuated. The difference in enhancement between CT and MRI may also be attributed to the different timing of these imaging studies. While post-contrast CT scans of the neck are usually acquired with a relatively short delay after contrast injection, post-contrast MRI take longer to acquire, reflecting the pattern of enhancement in which there is delayed wash-in and slow washout. Radiologically, the appearance is non-specific and variable consisting of a markedly enlarged lacrimal gland, cervical nodes with parotid and sub-mandibular glands, and intense enhancement of nodes and heterogeneous enhancement of salivary glands. Sometimes, MRI demonstrates serpentine signal-intensity-void areas, suggestive of vascular structures, within the lesions. The variability in the degree of enhancement on CT and MR images and the signal intensity on MR images may be due to the differing severity of fibrosis and vascular proliferation in the individual lesions. Abundant vascular proliferation may explain the presence of enhancement and signal-intensity-void structures on MR images [15].
Histopathological examination is essential to determine the diagnosis. The microscopic pattern is characterized by an atypical vascular proliferation associated with a variable chronic inflammatory cell infiltrate of lymphocytes and eosinophils and scattered lymphoid follicles. Characteristic finding is the presence of peculiar plump vacuolated endothelial cells of “epithelioid” or “histiocytoid” appearance lining the vascular lumina, which may even extend into the vascular spaces [4,9,10,16,17]. The attenuated endothelial cells of Kimura’s disease are pale and lack the dense eosinophilic cytoplasm found in the plump endothelial cells of ALHE [1]. Additionally, nuclear atypia is common in ALHE and is not seen in Kimura’s disease, while abundant fibrosis is common in Kimura’s disease and rarely seen in ALHE [18]. In our series, only 1 patient (case 2) had fibrosis during the chronic stage of the disease and repeat biopsy 3 years after the initial tissue diagnosis of ALHE.
Kimura's disease and ALHE are distinct. ALHE tends to have typical changes in endothelial cells described above with no lymphadenopathy and Kimura's disease tends to show typical lymphoid follicles in association clinically with lymphadenopathy and peripheral eosinophilia [19]. None of the cases in our study had typical lymphoid follicles supporting the diagnosis of Kimura’s disease. Alternately, they had the plump endothelial cells typical of ALHE.
The final diagnosis relies on distinguishing clinical-radiological and histopathological differences between many entities. Kimura’s disease is the most important differential diagnosis, others conditions include, Kaposi’s sarcoma, angiosarcoma, angiomatous lymphoid hamartoma, epithelioid hemangioendothelioma, bacillary angiomatosis, pyogenic granuloma, parasitic infections, eosinophilic granuloma, lymphoma, hemangioma, sarcoidosis, dermoid cyst and chalazion [1,3,10,11,18,20].
2021 Copyright OAT. All rights reserv
The most effective treatment of ALHE, as for other benign lesions, is complete surgical excision [11]. However, some adjunctive treatments have been suggested as systemic and intralesional steroid administration, interferon therapy, cryotherapy, laser therapy and topical application of tacrolimus have been used with success [21]. Recently, oral propanol and high dose of steroids have been proposed as a potential alternative therapy when complete excision of the lesion is not possible or in cases of recurrence after surgery [16,22]. Recurrence has been reported after incomplete excision [10]. However, most lesions regress spontaneously, and it is reasonable to observe the lesion for spontaneous regression after an incisional biopsy [10,23]. Good results have been reported with a stepwise multimodal treatment that combined low dose steroids and a cytotoxic agent as an initial treatment to reduce the lesion dimensions in preparation for surgical excision [24]. Six of the cases in our series had good prognosis. Meantime, one patient (case 6) required exenteration and progressed to death. We think this patient had an atypical presentation and clinical course, possibly complicated by secondary infection and sepsis as the chart notes indicated development of a suppurative process locally, lymphocytosis in the blood and fever.
In conclusion, we present seven cases of ALHE, an unusual lesion and very rare involvement of the orbit and ocular adnexa. The nonspecific clinical and radiologic findings and the characteristic histopathological examination allowed definitive diagnosis. The main treatment was excisional biopsy and the prognosis was good in 6 of 7 patients, confirming the benign nature of the disease.
Acknowledgement
The College of Medicine Research Center, Deanship of Scientific Research, King Saud University, Riyadh, Saudi Arabia, supported this work. None of the authors have commercial or proprietary interest in the material presented in this paper.
References
- McEachren TM, S Brownstein, DR Jordan, VA Montpetit, RL Font (2000) Epithelioid hemangioma of the orbit. Ophthalmology 107: 806-810.
- Sanchez-Orgaz M, Insausti-Garcia A, Gregorio LY, Duralde AA, Romero-Martin R (2014) Epithelioid hemangioma of the orbit or angiolymphoid hyperplasia with eosinophilia. Ophthal Plast Reconstr Surg 30: e70-72. [Crossref]
- Baker MS, Avery RB, Johnson CR, Allen RC (2012) Methotrexate as an alternative treatment for orbital angiolymphoid hyperplasia with eosinophilia. Orbit 31: 324-326. [Crossref]
- Fernandes BF, Al-Mujaini A, Petrogiannis-Haliotis T, Al-Kandari A, Arthurs B, et al. (2007) Epithelioid hemangioma (angiolymphoid hyperplasia with eosinophilia) of the orbit: a case report. J Med Case Rep 1: 30. [Crossref]
- Esmaili DD, Chang EL, O'Hearn TM, Smith RE, Rao NA (2008) Simultaneous presentation of Kimura disease and angiolymphoid hyperplasia with eosinophilia. Ophthal Plast Reconstr Surg 24: 310-311. [Crossref]
- Bangal S, Chitgopekar R, Gupta A, Karle R (2011) Orbital extension of supraorbital angiolymphoid hyperplasia with eosinophilia. Australas Med J 4: 111-113. [Crossref]
- Cook HT, Stafford ND (1988) Angiolymphoid hyperplasia with eosinophilia involving the lacrimal gland: case report. Br J Ophthalmol 72: 710-712. [Crossref]
- Sheren SB, Custer PL, Smith ME (1989) Angiolymphoid hyperplasia with eosinophilia of the orbit associated with obstructive airway disease. Am J Ophthalmol 108: 167-169. [Crossref]
- Acocella A, Catelani C, Nardi P (2005) Angiolymphoid hyperplasia with eosinophilia: a case report of orbital involvement. J Oral Maxillofac Surg 63: 140-144. [Crossref]
- Azari AA, Kanavi MR, Lucarelli M, Lee V, Lundin AM, et al. (2014) Angiolymphoid hyperplasia with eosinophilia of the orbit and ocular adnexa: report of 5 cases. JAMA Ophthalmol 132: 633-636. [Crossref]
- Archer KF, Hurwitz JJ, Heathcote G (1991) Orbital angiolymphoid hyperplasia with eosinophilia. Presentation as chalazion. Ophthal Plast Reconstr Surg 7: 208-221. [Crossref]
- Sanchez-Acosta A, Moreno-Arredondo D, Rubio-Solornio RI, Rodriguez-Martinez HA, Rodriguez-Reyes AA (2008) Angiolymphoid hyperplasia with eosinophilia of the lacrimal gland: a case report. Orbit 27: 195-198. [Crossref]
- Shields C, Shields J, Glass R (1990) Bilateral orbital involvement with angiolymphoid hyperplasia with eosinophilia (Kimura´s disease). Orbit 9: 89-95.
- Barakova D, Sach J, Kuchynka P, Redinova M, Kocur I (2002) [Angiolymphoid hyperplasia with eosinophilia with bilateral involvement of the lacrimal glands]. Klin Monbl Augenheilkd 219: 376-379. [Crossref]
- Bostad L, Pettersen W (1982) Angiolymphoid hyperplasia with eosinophilia involving the orbita. A case report. Acta Ophthalmol 60: 419-426. [Crossref]
- Alder B, Proia A, Liss J (2013) Distinct, bilateral epithelioid hemangioma of the orbit. Orbit 32: 51-53. [Crossref]
- Cunniffe G, Alonso T, Dinares C, Medina FJ, Medel R (2014) Angiolymphoid hyperplasia with eosinophilia of the eyelid and orbit: the Western cousin of Kimura's disease? Int Ophthalmol 34: 107-110. [Crossref]
- Hidayat AA, Cameron JD, Font RL, Zimmerman LE (1983) Angiolymphoid hyperplasia with eosinophilia (Kimura's disease) of the orbit and ocular adnexa. Am J Ophthalmol 96: 176-189. [Crossref]
- Chun SI, HG JI (1992) Kimura's disease and angiolymphoid hyperplasia with eosinophilia: clinical and histopathologic differences. J Am Acad Dermatol 27: 954-958. [Crossref]
- Buggage RR, Spraul CW, Wojno TH, Grossniklaus HE (1999) Kimura disease of the orbit and ocular adnexa. Surv Ophthalmol 44: 79-91. [Crossref]
- Wolff D, Andree H, Hilgendorf I, Casper J, Freund M, et al. (2008) Sirolimus in combination with tacrolimus in allogeneic stem cell transplantation--timing and conditioning regimen may be crucial. Biol Blood Marrow Transplant 14: 942-943. [Crossref]
- Moss HB, Sines DT, Blatt J, Dutton JJ, Proia AD (2012) Epithelioid hemangioma responsive to oral propranolol. Ophthal Plast Reconstr Surg 28: e88-90. [Crossref]
- Lin B, Tan SH, Looi A (2008) Angiolymphoid hyperplasia with eosinophilia of the eyelid with spontaneous regression. Ophthal Plast Reconstr Surg 24: 308-310. [Crossref]
- Krema H, El-Bolkainy N (2014) A stepwise multimodality treatment of diffuse angiolymphoid hyperplasia of the orbit. Orbit 33: 75-77. [Crossref]