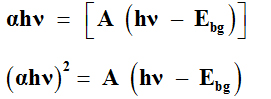Abstract
Nanomaterial- Zinc oxide (ZnO) has been synthesized using Zinc Nitrate Hexahydrate as precursor by Sonochemical Method. The as-synthesized ZnO was characterized to study their optical, morphological and physical properties. Fourier Transform-Infra Red Spectroscopy analysis was done for identification of functional group, Scanning Electron Microscopy- Energy Dispersive X-Ray Analysis for Morphology and Size and elemental confirmation, Transmission Electron Microscopy for determination of particle size and shape and X-Ray Diffraction for the identification of crystal structure. The material developed was used in a membrane filtration technology for degradation of selected pesticide. Pesticides(Chlorpyrifos) taken at fixed concentration (5 ppm) in a solvent and then stimulated effluents were subjected to photocatalytic degradation and allow to pass through the membrane filtration assembly under low pressure using cellulose acetate mixed polymeric membrane discs. Samples were analyzed by UV-VIS spectroscopy for the assessment of degradation of Chlorpyrifos. Results shows that ZnO alone show low degradation efficiency as compared to membrane filtration. When combined both the approaches (ZnO + Membrane filtration) there is steep decline in the absorption peak. Nanobased membrane filtration technology design and developed were found effective and efficient for the degradation of pesticides as compared to photocatalytic degradation carried out using ZnO.
Key words
zinc oxide, pesticides, membrane filtration, photocatalytic degradation, chlorpyrifos.
Introduction
Pesticide pollution emerges as the serious environmental concern. Degradation and mineralization of pesticide contaminants has becomes the key concern of scientific community. The main cause of pesticide pollution is the excessive use of pesticide, by-products and intermediated form during the degradation [1] . This persistent pollution mainly arises due to incomplete degradation by the conventional techniques, because of their high chemical stability and low biodegradability and high persistence in the atmosphere [2,3].
Photocatalytic degradation by semiconducting nanoparticles is mainly considered as potential and effective technology for the mineralization of pesticide into environmental friendly compounds [4]. Nanoparticles have emerged as sustainable alternatives to conventional bulk materials, as robust, high surface area heterogeneous photo-catalysts and catalyst supports [5]. The nano-sized particles have high surface to volume ratio which increase the exposed surface area of the active component of the catalyst, enhances the contact between reactants and catalyst [6]. Surface area plays an important role in the photocatalytic activity, due to that focus hadbeen shifted towards the semiconducting nanomaterial because of their high surface to volume ratio [7]. A high surface-to-volume ratio (SVR) is required for a contaminant molecule to be adsorbed on to the surface of photocatalyst for the redox reactions (Oxidation and Reduction) to occur for the complete degradation/mineralization of the contaminant [8,9]. Higher the catalytic surface area, higher will be the adsorption of target molecules on the surface, leading better photocatalytic activity and complete degradation of contaminant [10]. The potential application of semiconducting materials for solar energy conversion, production and remediation is now realized by the scientific community [11]. TiO2 and ZnO are found to be best among all photocatalyst because of their wide band gap energy, biological and chemical inertness, ease of synthesis and applicability [12]. Among various semiconducting nanomaterials, zinc oxide (ZnO) is a distinctive wurtzite n-type semiconductor with a wide direct band gap of 3.37 eV and a high exciton binding energy (60 meV) at room temperature [13]. The complete degradation of organic pollutants is not possible by conventional approaches such as anaerobic digestion, activated sludge digestion, physicochemical treatment, and air stripping as they only transfers the contaminants from one phase to another. At present, there are several methods available for pesticide removal such as advanced oxidation processes, photocatalytic degradation (heterogeneous catalysis), combined photo-Fenton (homogeneous catalysis), biological oxidation (microbial degradation), aerobic degradation, membrane filtration (by nanofiltration membranes), coagulation, solid phase extraction, ozonation, fluid extraction and adsorption [14]. Also the main method of pesticide disposal is incineration, which is very expensive and generally not available in developing nations. Thus there is need for the alternative method for complete mineralization of pesticides into the environmental friendly compounds. Out of different methods, Advance Oxidation Process (AOP) using nano based semiconductors as a photocatalyst for the degradation of pesticides is considered as most efficient, promising and environmental friendly technique [15]. Among Advance Oxidation Processes, heterogeneous photocatalysis or semiconductor mediated photocatalysis appears the most emerging destructive technology for the remediation of organic contaminants [10,16]. The drawback associated with the photocatalysis is the retrieval of photocatalyst after the reaction, because of this the alternative to this approach which can degrade and filtrate both at the same time [17]. The use of membrane filtration for the pesticide removal is not new but the approach combine with photocatalysis is novel for the efficient degradation. Membrane filtration is gaining importance in pesticide degradation; generally nanofiltration membrane is utilized for the same [18,19]. The main disadvantage of photocatalytic degradation is to retrieve the photocatalyst after degradation is over; the very quantity of catalyst is present in the treated effluent even after centrifugation [5]. The objective behind using the membrane filtration is to recover the catalyst after the degradation purpose. The study of reproducibility of the photocatalyst can be done after drying. There are very limited studies till date considering the recycling of catalyst material [20].
The present study focuses on the synthesis of ZnO nanoparticles by sonochemical method using Zinc Nitrate Hexahydrate (Zn (NO3)2. 6H2O) as a precursor of zinc and the characterization of ZnO by different characterizing tools. Sonochemical method is based on the acoustic cavitation from the continuous formation and collapse of the bubbles in a liquid [21]. This method is basically known to produce nanocrystals under the appropriate condition. Technical grade Chlorpyrifos (EC 50%) is taken as a model pesticide for the degradation studies. Mixed cellulose acetate polymeric membrane discs is used for the filtration purpose under low pressure. After photocatalytic degradation the treated effluent is allowed to pass through the membrane in a filtration assembly under low pressure. UV-Vis spectroscopy is used to study the degradation efficiency of the selected pesticide. Reaction process was optimized by taking the varying concentration of catalysts and pesticide.
Structure of chlorpyrifos and mode of action
Chlorpyrifos is basically an Organophosphate group of insecticide that inhibits the acetyl cholinesterase enzyme associated with the functioning of nervous system. It is wide spectrum insecticide thoroughly used to kill wide range of insect’s pest in agriculture worldwide [22]. OP compounds are basically target the nervous system; sometime it affects the non-target group of organisms also [23]. It is highly toxic for aquatic organisms even the low concentration like parts per trillion. Symptom associated with the chlorpyrifos poisoning including nausea, vomiting, diarrhea, headache, convulsions, coma and death in severe conditions [24]. The general structural formula of chlorpyrifos is O,O-diethyl O-3,5,6-trichloro-2 pyridylphosphorothioate (Figure 1).
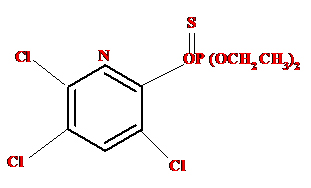
Figure 1. Structure of Chlorpyrifos
Materials and methods
Chemicals
Analytical grade Zinc Nitrate Hexahydrate (Zn (NO3)2. 6H2O), Sodium Hydroxide (NaOH) (Sigma Aldrich) pellets were purchased and used without further purification. Zinc oxide nanoparticles were synthesized using sonochemical chemical method using zinc nitrate hexahydrate as precursors. Sonochemical method was selected for the synthesis of ZnO nanoparticle because it provides nanoparticle of appropriate size. Commercially available technical grade Chlorpyrifos (EC 50%) is used for the degradation study.
Experimental method
All the reactions were carried out at room temperature under ambient conditions.
Synthesis of ZnO nanoparticles: In a typical reaction, 0.5 M of Zinc Nitrate Hexahydrate (Zn (NO3)2. 6H2O) was dissolved in 100 ml distilled water under continuous stirring. 0.5 M NaOH is prepared in different reaction vessel and stir it continuously until it gets dissolved. NaOH solution was added drop wise to the Zinc nitrate solution under continuous stirring until white precipitates formed. Seal the vessel and reaction is allowed to continue for 3 hours of vigorous stirring. After that the solution was kept in Ultra-sonication for 1 hour at 37°C (20-40 kHz). After sonication, the precipitates was centrifuged and washed with double distilled water several times and finally with ethanol to completely remove all the impurities. The precipitates were dried at 100°C for complete reduction into ZnO nanoparticle. Grind the powder in mortar and pestle to obtain the fine powder of ZnO and store it for further experiment.
Customized photocatalytic reactor and filtration assembly
All the photocatalytic experiment was conducted in a rectangular borosil glass reactor that is customized in fixed dimensions. Two UV lamp of 11W (Philips) having wavelength of 365 nm were used as a source of UV in the customized photo reactor fitted over the roof of the reactor. Few holes were drilled on the side of the reactor chamber to maintain the proper aeration and temperature. All the process was carried out under strict lab conditions. A filtration assembly equipped with pressure pump is utilized for the membrane filtration reaction simultaneously (Figure 2).
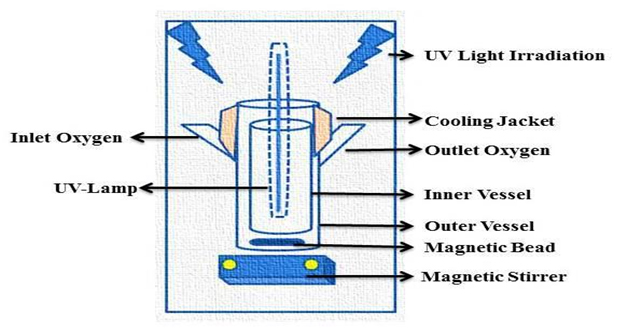
Figure 2. Schematic representation of Photocatalytic Reactor
Photocatalytic degradation studies
In present work, photocatalytic degradation of pesticide has been measured under UV light irradiation in aqueous dispersion of synthesized ZnO to find the optimum reaction condition for a photocatalytic membrane filtration reactor (PMFR). The advantage of using the mixed cellulose acetate (MCA) polymeric membrane is to recover the ZnO photocatalyst after filtration. Low pressure ultrafiltration using hollow-fiber MCA membrane was applied for present study. The photocatalysis result indicates that the ZnO exhibits good degradation efficiency but when it combines with the membrane filtration the degradation efficiency increases manifolds. Dark adsorption studies were conducted with chlorpyrifos to correlates the adsorption and degradation results (i.e. photolysis and photocatalysis). The optimized concentration of pesticide solution was prepared in distilled water. The amount of catalyst was fixed during the whole reaction whereas the concentration of the pesticide was kept varying. A volume of 250 ml of stock solution (5 ppm) is prepared and taken in the reactor and fixed amount of catalyst was added. The suspension was first stirred under total dark condition for half an hour to reach the adsorption maximum. The stirring rate of 500 rpm was maintained for all the experiments. Now the solution was subjected under UV irradiation with vigorous stirring in the reactor chamber by magnetic stirrer for fixed time period. An aliquot of 5-10 ml sample were taken at regular time intervals with the help of sample syringe. The sample was then pass through the 0.45 Mm membrane filter to remove the particle and agglomerates. At last, the sample were pass through the membrane filtration assembly under low pressure after the degradation was over. The degradation efficiency of pesticide was studied using the UV-VIS spectrophotometer.
Result and discussion
Characterization
The X-Ray diffraction patterns were recorded on Bruker X-Ray Diffractometer using graphite filtered CuK radiation (λ=1.54 Å) at 40 KV with scanning rate of 3/min (from 2θ=20-80°). Optical absorption spectra were recorded on a UV-Vis spectrometer (Shimadzu). Size and Morphology of the particles were determined by the 200 KeV Transmission Electron Microscope (TECNAI 200 Kv TEM- Fei, Electron Optics). Fourier Transmission Infrared spectroscopy was performed to determine the functional group of the obtained products (Perkin Elmer- Spectrum RX-IFTIR). Elemental Detection of the particle was carried out separately using SEM-EDAX (JEOL JSM 5600,EDS Model: INCA Oxford).
Fourier Transform-Infra Red Spectroscopy (FT-IR): FT-IR studies were performed to verify the bond structure and identification of associated functional groups of as-synthesized ZnO nanoparticles using optimized parameters. The infrared absorption spectra of ZnO nanoparticles were observed in the 4000-400 cm-1 wave-number range (Figure 3). The band located at 436 cm-1 attributed to the Zn–O stretching mode of the ZnO lattice [25]. The bands at 3466 cm-1 attributed to the O-H mode of vibration. C=O exhibiting strong asymmetric mode of vibration between 1633 and 1558 cm-1. The symmetric stretching occurs between 1508 and 1378 cm-1 because of presence of C-O. C-O-C peak is also present there.
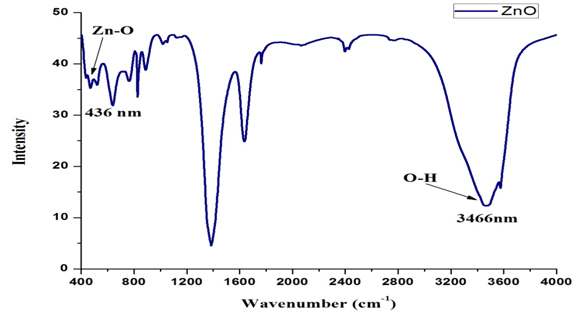
Figure 3. FT-IR spectra of ZnO.
Scanning Electron Microscopy- Energy Dispersive X-Ray Analysis (SEM-EDX)Morphological structure of as-synthesized ZnO was confirmed by SEM and the elemental composition of the particles was confirmed by EDAX [26]. The SEM-EDS micrograph of ZnO nanoparticles is shown in Figure 4. The EDAX spectra confirm the presence of Zn and O and no other impurity were detected.
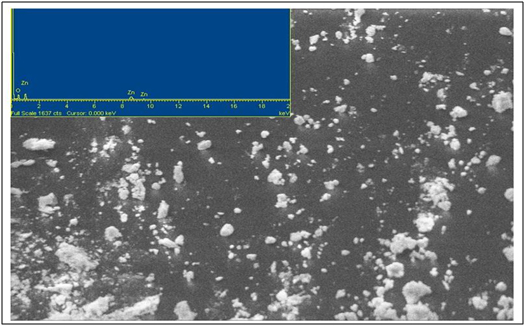
Figure 4. SEM Micrograph of as-synthesized ZnO (Inset- EDS spectra)
X-Ray Diffraction (XRD): Figure 5 shows the XRD spectrum of pure Zinc Oxide. The narrow and sharp diffraction peak indicates the particles have good size and crystallinity [27]. The diffraction angle of 2θ scanned in the range of 20-80°. The representative peaks appeared at 31.71, 34.37, 36.19, 47.49, and 56.51, revealed the reflecting planes at (100), (002), (101), (110), and (200) respectively. All the diffraction peaks shows the strong resemblance with the reported Joint Committee on Powder Diffraction Standards (JCPDS) belonging to hexagonal wurtzite crystal phase of ZnO.
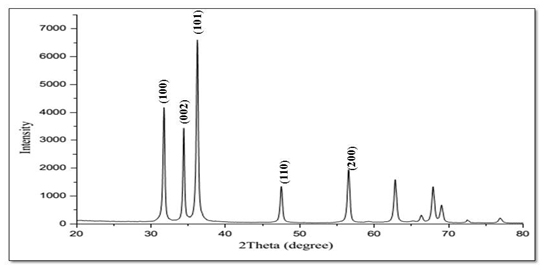
Figure 5. XRD Diffractogram of ZnO.
Transmission Electron Microscope (TEM): To know the actual size and morphology of the particles, samples were systematically analyzed by TEM. The TEM image of ZnO shows the formation of spherical aggregates with dimension of ~50 nm. The actual size of the nanoparticles was found to be around 26 nm. TEM image confirms that the morphology of nanoparticle were spheroid (Figure 6) [28,29].
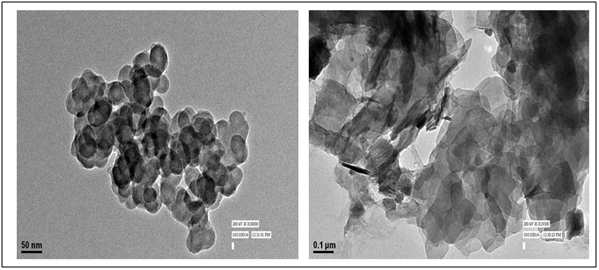
Figure 6. TEM Micrograph of ZnO
2021 Copyright OAT. All rights reserv
Ultraviolet- Diffuse Reflectance Spectroscopy (UV-DRS): The sample shows strong excitonic peaks with the same absorbance intensity at wavelength of 380 nm. The optical band gap of the powdered samples was characterized by using DRS [8]. The optical band gap of synthesized material was found to be 3.23 eV which is very close to the bulk ZnO [30] calculated using Kubelka-Munk Transformation (Figure 7 a & b).
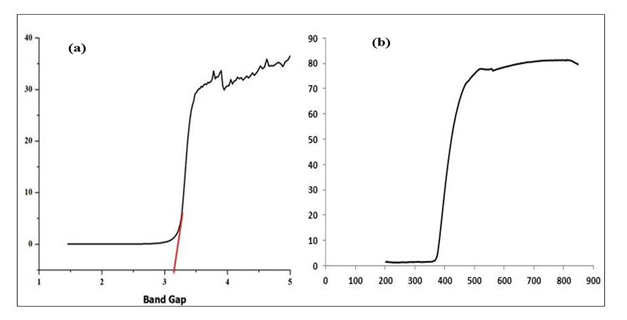
Figure 7. a) Optical bang gap of ZnO; b) Diffuse Reflectance Spectra of as-synthesized ZnO
The energy bang gap of the ZnO was determined using the following relationship:
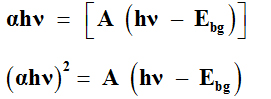
Where, hν is the energy of photon, α is the absorption coefficient, Ebgis the energy band gap and A is the constant.
Photocatalytic-Membrane Filtration Studies: For photolysis studies, required quantity of 5 ppm chlorpyrifos (EC 50%) was stirred for many hours after the addition of catalyst under dark condition. As soon as, the photocatalyst was added to the solution, pesticide starts to adsorb on the surface of the catalyst. It can be easily understood that there is a difference in the degradation efficiency after using membrane filtration as compared to ZnO mediated photocatalysis [31-34]. The initial absorption spectra of 5 ppm chlorpyrifos and the final absorption spectra after filtration can be easily understood by Figure 8 (a). It is concluded that the maximum degradation is achieved in 60 minutes; the plot shows decrease in degradation efficiency after 60 minutes. The comparative plot of initial, photolysis, 60 minutes of degradation and final filtration is shown in Figure 8 (c). Thus, it was confirmed from the spectrophotometric studies which shows that the membrane filtration is a good approach when combine with the photocatalysis for the degradation of pesticides. It can also be utilized for the degradation of other contaminants as well [35,36].
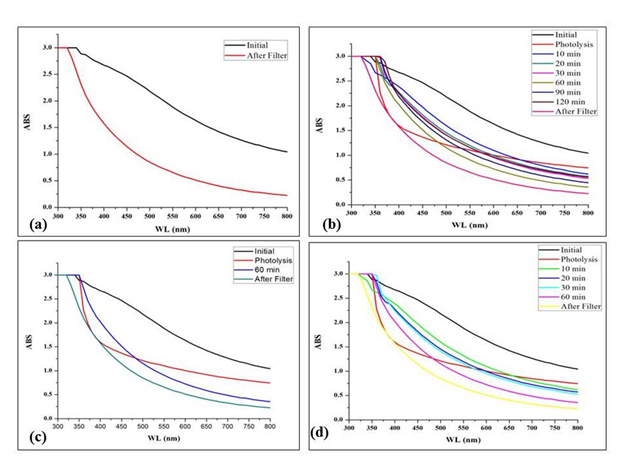
Figure 8. Plot showing the UV absorption spectra of rapid degradation of chlorpyrifos at before and after filtration (a, b, c & d).
Conclusion
Zinc oxide nanoparticle has been successfully synthesized by sonochemical method, characterized and used as a photocatalyst for the degradation of pesticide. The results confirms the synthesis of ZnO under the nano range (1-100 nm) having spherical shape and hexagonal wurtzite crystal structure. The photocatalytic results of the as-synthesized ZnO indicates that the ZnO exhibit good photocatalytic efficiency under UV light irradiation but when combine with membrane filtration; degradation rate increases several time as compared to ZnO mediated photocatalysis. The results confirmed that at 5 ppm, maximum degradation achieved in 60 minutes and further degradation is achieved after membrane filtration. Present study confirmed that the Membrane filtration combined with photocatalytic degradation is efficient and time savvy technique for the degradation of pesticide compounds.
Acknowledgement
This work was supported by the University Grant Commission-Maulana Azad National Fellowship under Grant [MANF/2012-13/MUS/MAD/12852]. The authors are also thankful to Central Instrumentation Facility, CUG, Gandhinagar, SAIF- Punjab University, Chandigarh, SAIF- AIIMS, New Delhi, Dr. Uday Deshpande and Dr. D.M. Phase, UGC-CSR, Indore for extending analysis facility.
References
- Biziuk M, Przyjazny A, Czerwinski J, Wiergowski M (1996) Occurrence and determination of pesticides in natural and treated waters. J Chromatogr A 754: 103-123. [Crossref]
- Bootharaju MS, Pradeep T (2012) Understanding the degradation pathway of the pesticide, chlorpyrifos by noble metal nanoparticles. Langmuir 28: 2671-2679. [Crossref]
- Colina-márquez J, Machuca-martínez F, Salas W (2013) Enhancement of the potential biodegradability and the mineralization of a pesticides mixture after being treated by a coupled process of TiO 2 -based solar photocatalysis with constructed wetlands 190: 181-190.
- Chauhan R, Kumar A (2012) Photocatalytic studies of silver doped ZnO nanoparticles synthesized by chemical precipitation method 546-553.
- FujishimaA, Zhang X, Tryk D (2007) Heterogeneous photocatalysis: From water photolysis to applications in environmental cleanup. International Journal of Hydrogen Energy 32: 2664-2672.
- BansalP, Dhir A, Prakash NT, Sud D (2011) Environmental remediation of wastewater containing azo dyes with a heterostructured nanophotocatalyst 50: 991-995.
- Chen W, Qi D, Gao X, Thye A, Wee S (2009) Progress in Surface Science Surface transfer doping of semiconductors. Progress in Surface Science 84: 279-321
- Janott A, Walle CGV (2009) Fundamentals of zinc oxide as asemiconductor. Rep Prog Phys. 72: 26501
- Herrmann J (1999) Heterogeneous photocatalysis: fundamentals and applications to the removal of various types of aqueous pollutants. Catalysis Today 53115-: 129.
- Chong MN, Jin B, Chow CW, Saint C (2010) Recent developments in photocatalytic water treatment technology: a review. Water Res 44: 2997-3027. [Crossref]
- BangalM, Ashtaputre S, Marathe S, Ethiraj A, et al. (2005) Semiconductor nanoparticles. Hyperfine Interactions, 160: 81-94.
- Beydoun D, Amal R, Low G, Mcevoy S (2000) Role of nanoparticles in photocatalysis 439-458.
- BaruahS, Pal SK, Dutta J (2012) Nanostructured Zinc Oxide for Water Treatment. Nanoscience and Technology-Asia 2: 90-102.
- Bhatkhande DS, Pangarkar VG, Beenackers M (2002) Photocatalytic degradation for environmental applications - a review. Journal of Chemical Technology & Biotechnology 77: 15
- Devipriya S, Ã S Y (2005) Photocatalytic degradation of pesticide contaminants in water, 86: 309-348.
- Tayade RJ, Surolia PK, Kulkarni RG, Jasra RV (2007) Photocatalytic degradation of dyes and organic contaminants in water using nanocrystallineanatase and rutile TiO 2, 455.
- Daneshvar N, Hejazi, MJ, Rangarangy B, Khataee AR (2004) Photocatalytic Degradation of an Organophosphorus Pesticide Phosalone in Aqueous Suspensions of Titanium Dioxide Photocatalytic Degradation of an Organophosphorus Pesticide Phosalone in Aqueous Suspensions, Journal of Environmental Science and Health, Part B: Pesticides, Food Contaminants, and Agricultural Wastes, 37-41.
- Gallucci F, Basile A (2011) Introduction - A review of membrane reactors, 1-61.
- Kimura S, Kodama S, Sekiguchi H (2013) Development of photocatalytic reactor having light source inside by electrical discharge: 4-7.
- KonstantinouI K, Albanis TA (2002) Photocatalytic transformation of pesticides in aqueous titanium dioxide suspensions using artificial and solar light. Intermediates and Degradation Pathways 1310: 1-17.
- Kiran K (2013) Detection of chlorpyrifos pesticide in various water samples using gold nanoparticles, 2319-2322.
- Fishel FM (2011) Pesticide Toxicity Profile: Organophosphate Pesticides.
- VermaA, Dixit D (2012) Photocatalytic degradability of insecticide Chlorpyrifos over UV irradiated Titanium dioxide in aqueous phase 3: 743-755.
- Gowthamana P, Sarojab M, Venkatachalamb M, Deenathayalana J, Senthil TS (2011) Photocatalytic degradation of methelene blue dye using hydrothermally synthesized ZnO nanorods, Optoelectronics and Advanced Materials - Rapid Communications 5: 1307-1311.
- Ali MA, Idris MR, Ali MEQ (2013) Fabrication of ZnO nanoparticles by solution combustion method for the photocatalytic degradation of organic dye. Journal of Nanostructure in Chemistry 3: 36.
- Hsieh C (2007) Spherical Zinc Oxide Nano Particles from Zinc Acetate in the Precipitation Method 31-34.
- Hu Z, Oskam G, Searson PC (2003) Influence of solvent on the growth of ZnO nanoparticles. J Colloid Interface Sci 263: 454-460. [Crossref]
- Ha T, Canh TD, Tuyen NV (2013) A Quick Process for Synthesis of ZnO Nanoparticles with the Aid of Microwave Irradiation, ISRN Nanotechnology.
- Alam SN (2012) SEM, EDX & XRD of Zinc Oxide Nanostructures Synthesized by Zinc Oxidation. Microscopy and Analysis 26: 11-14.
- AshokkumarM (1998) An overview on semiconductor particulate systems for photoproduction of hydrogen. International Journal of Hydrogen Energy 23: 427-438.
- BecheriA, Durr M, Nostro PD, Baglioni P (2008) Synthesis and characterization of zinc oxide nanoparticles: application to textiles as UV-absorbers. J Nanopart Res 10: 679-689.
- Choi E, Cho IH, Park J (2004) The effect of operational parameters on the photocatalytic degradation of pesticide. J Environ Sci Health B 39: 53-64. [Crossref]
- DaraeiH, Maleki A, Mahvi AH, Zandsalimi Y, AlaeiL (2013) Synthesis of ZnO nano-sono-catalyst for degradation of reactive dye focusing on energy consumption: operational parameters influence, modeling, and optimization. Desalination and Water Treatment: 37-41.
- Elamin N, Elsanousi A (2013) Synthesis of ZnO Nanostructures and their Photocatalytic Activity 1: 32-35.
- Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 238: 37-38. [Crossref]
- Singh A, Dutta DP, Roy M (2013) Sonochemical synthesis , characterization , and photocatalytic properties of Bi 2 2 x Sbx WO 6. Nanorods.