Aim: To elucidate factors associated with a poor neonatal prognosis in preterm placental abruption.
Methods: A multicenter retrospective study was conducted. A total of 247 cases with preterm placental abruption during 2009, derived from an electronic database constructed by the Japan Society of Obstetrics and Gynecology, were investigated as the subjects of this study, and were divided into three groups: very preterm (VPT, n=66) was delivered at 28 to 31 gestational weeks, preterm (n=56) was at 32 and 33 weeks, and late preterm (LPT, n=125) was at 34 to 36 weeks. Risk factors, clinical course, and neonatal/infantile outcomes were compared. Poor neonatal outcome was defined as stillbirth, or neonatal and infantile death, or cases with neurological disability at 2 years of age.
Results: The number of subjects who received tocolytic therapy was significantly higher in the VPT group as compared with the LPT group. It was higher in the VPT group even after excluding the 57 stillbirths. Multiple logistic regression analysis revealed that poor prognostic factors were umbilical arterial pH (Δ=0.1) (0.17 [0.069-0.417], p<0.0001) and tocolytic therapy (28.5 [2.014-403.9], p<0.0132).
Conclusion: Administration of tocolytic agents is a possible factor in a poor neonatal prognosis in preterm placental abruption. This finding may suggest that differential diagnosis could improve the prognosis.
neonatal outcome, placental abruption, preterm delivery, tocolytic therapy
Placental abruption is a disorder that can result in a serious outcome for both the mother and child. It can still result in maternal death in Japan, and it accounts for one-third of perinatal deaths [1,2]. Therefore, early diagnosis and management are essential. However, this disorder is expressed in a wide range of forms (onset can be relatively gradual in some cases and very dramatic after retro-placental hematoma in other cases), and there is still no effective strategy for diagnosis and prevention. In a previous study, we found that the most effective markers for predicting a poor perinatal prognosis in placental abruption were Fetal Heart Rate (FHR) monitoring findings, particularly prolonged deceleration and repeated late deceleration [3]. Consequently, our findings suggested the importance of early diagnosis by means of fetal heart monitoring and pregnancy termination to improve the prognosis. However, there is no reliable evidence regarding prediction and prevention of onset. The reason for this is the fact that the etiology of this disorder has not been adequately elucidated.
Epidemiologic investigations have revealed many risk factors for onset of placental abruption. They include primiparity, high maternal age at primiparity, male fetus, smoking habit, pregnancy-induced hypertension (PIH), threatened premature labor, chorioamnionitis, fetal growth restriction, and polyhydramnios [4-6]. Those investigations have suggested the involvement of an immunological mechanism or genetic mechanism [7,8]. Pathologically, this disorder can be viewed as occurring due to failure of adhesion of the placental villi and uterine decidua for some reason. Ananth et al. [3] hypothesized that there were two pathways underlying the mechanism by which adhesion fails [4]. They suggested the presence of an acute process seen in the second trimester and a chronic process observed in the late third trimester. It is understood that inflammatory cytokines are involved in the former, while the latter is associated with capillary endothelial injury. Considering the pathological causes of this disorder, an investigation that takes into account the cause and timing of onset may be necessary. The present study, which was limited to cases of preterm onset and excluded full-term onset, was conducted to uncover factors associated with a poor prognosis by stratifying patients by week of gestation.
A multicenter retrospective case-cohort study was conducted after being approved by our Institutional Review Board (certification number 2301). This study was analyzed as a subcohort study from our previous report [3]. We reviewed the computerized chart records from the database of the Japan Perinatal Registry Network, which is managed by the Japan Society of Obstetrics and Gynecology, during the period from January 1 to December 31 in 2009. This database, derived from 131 tertiary and/or secondary perinatal hospitals, including 76 university hospitals, 11 national hospitals, 11 Red Cross hospitals, and 33 other hospitals, covered 76,113 births after 22 gestational weeks, 7.1% of all deliveries (1,073,680 births in same period) in Japan. In this database, 570 pregnant women were diagnosed with placental abruption during this study period. The following exclusion criteria were included: placental abruption occurred at less than 28 or over 36 gestational weeks, unclear estimated confinement date provided by last menstruation period supported by early trimester ultrasound, twin gestation, chronic abruption oligohydramnios sequence [9], and cases lost to follow-up of the infant at 2 years of age. The remaining 247 cases were investigated as the subjects in this study, and were divided into three groups: very preterm (VPT, n=66) was delivered at 28 to 31 gestational weeks, preterm (PT, n=56) was delivered at 32 and 33 weeks, and late preterm (LPT, n=125) was delivered at 34 to 36 weeks. The distribution of study subjects is shown in Figure 1. Maternal background factors, risk factors for placental abruption, clinical course factors, i.e., initial symptom, clinical signs confirming abruption, treatment, delayed time from onset to admission or from onset to delivery, and maternal ambulance transfer to perinatal medical center, were compared between these three groups. Perinatal and neonatal/infantile outcomes were also compared.
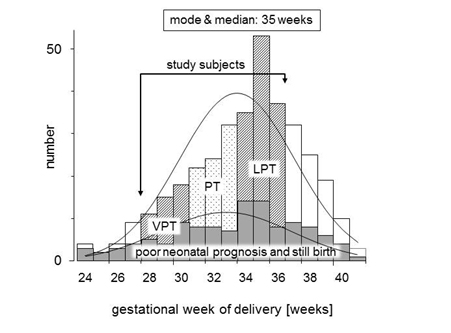
Figure 1. Distribution pattern of subjects in this study by gestational week.
Placental abruption was defined as cases with one or more of the following symptoms: genital bleeding, abdominal pain, uterine tenderness, retro-placental hematoma confirmed by ultrasound, with/without abnormal FHR monitoring, and confirmation by clear evidence of placental separation during pregnancy, i.e., retro-placental hematoma or bloody amniotic fluid after birth [10]. Abnormal FHR monitoring included abnormal baseline heart rates less than 110 beats per minute (bpm) or over 160 bpm, and/or presence of recurrent late decelerations or severe variable decelerations, and/or prolonged decelerations, and/or loss of FHR variability.
Obstetrical and other complications were defined as follows: PIH was defined according to the criteria of the National High Blood Pressure Education Program [11], chronic hypertension was defined as hypertension before pregnancy or diagnosed before 20 gestational weeks, and previous abruption was defined as cases that experienced placental abruption in a previous pregnancy. Obstetric Disseminated Intravascular Coagulation (DIC) score was calculated with a unique scoring system previously reported [12,13], which is a very popular scoring system in Japan. The patient is regarded as having DIC when this score is 8 or more. Threatened premature labor was defined as one or more symptoms of atypical genital bleeding, labor pain, and cervix dilatation. In cases of threatened premature labor, tocolytic therapy was needed. Usage of tocolytic agents was as follows. Ritodrine hydrochloride was administered intravenously at an initial dose of 50 μg/minute, increased by 50 μg/minute every 10-20 minutes up to a maximum dose of 200 μg/minute, until labor pains disappeared. Poor neonatal outcome was defined as stillbirth or Intrauterine Fetal Death (IUFD), or neonatal and infantile death, or cases with neurological disability at 2 years of age. Favorable neonatal outcome was defined as cases without any adverse neurological outcomes in an infantile survey at 2 years of age.
Statistical analyses were performed with a computer program: Statflex 6.0 (Artech Co., Ltd., Osaka, Japan. URL: http://www.statflex.net/). Values are shown as means ± Standard Deviations (SD) or the number with percentage. Analysis of variance with post hoc test or kai square test was performed as an evaluation in the mean difference or in the proportional difference, respectively. A p value less than 5% was considered to be significant. Multiple logistic regression analysis was performed to clarify the most influencing factors for poor neonatal/infantile outcome. Relative risk was presented as the odds ratio with 95% confidence intervals (OR [95%CI]).
A frequency distribution map for the full analysis set (n=570) in this study, and for subjects (n=247) who met the entry criteria and did not violate the exclusion criteria, is shown in Figure by gestational week. The breakdown of the 247 subjects was 66 subjects (25.8%) in the VPT group, 56 subjects (25.4%) in the PT group, and 125 subjects (48.8%) in the LPT group (Table 1). There were no significant differences between the groups in terms of maternal background factors such as age, primiparity, and presence of risk factors. In terms of information on the course of pregnancy, the number of subjects who received tocolytic therapy was significantly higher in the VPT group as compared with the LPT group. It was higher in the VPT group even after excluding the 57 stillbirths (VPT group: 16 subjects, PT group: 11 subjects, LPT group: 30 subjects). With respect to perinatal information, the number of subjects with an Apgar score <7 at 1 minute increased as the gestational week decreased. However, no significant difference was seen for Apgar scores at 5 minutes. The neonatal prognosis excluding stillbirths was poor in 10/50 subjects (20.0%) in the VPT group, 4/45 subjects (8.9%) in the PT group, and 6/95 subjects (6.3%) in the LPT group, i.e., it was significantly higher in the VPT group.
Table 1. Clinical characteristics and comparisons of the study subjects.
|
VPT (n=66) |
PT (n=56) |
LPT (n=125) |
p value |
Age |
32.1 ± 5.6 |
33.1 ± 4.3 |
31.5 ± 4.9 |
n.s.* |
Primipara |
32 (48.5%) |
26 (46.4%) |
66 (52.8%) |
n.s.† |
Risk factors |
|
|
|
|
PIH |
18 (27.3%) |
17 (30.4%) |
24 (19.2%) |
n.s.† |
Chronic hypertension |
12 (18.2%) |
7 (12.5%) |
10 (8.0%) |
n.s.† |
Smoking habit |
6 (9.1%) |
9 (16.1%) |
13 (10.4%) |
n.s.† |
Previous abruption |
3 (4.5%) |
1 (1.8%) |
1 (0.8%) |
n.s.† |
Trauma |
0 (0%) |
0 (0%) |
1 (0.8%) |
n.s.† |
Delay time [hours] |
|
|
|
|
onset to admission |
4.0 ± 3.9 |
3.4 ± 2.3 |
3.2 ± 3.1 |
n.s.* |
onset to delivery |
4.2 ± 3.9 |
3.7 ± 3.2 |
4.3 ± 6.0 |
n.s.* |
Maternal transfer |
46 (70.0%) |
44 (78.6%) |
67 (53.6%) |
n.s.† |
Tocolysis before delivery |
|
|
|
|
including IUFD |
24 (36.4%) |
13 (23.2%) |
17 (13.6%) |
0.001† |
excluding IUFD |
21 (42.0%) |
12 (26.7%) |
17 (17.9%) |
0.007† |
Abnormal FHR monitoring |
32 (48.5%) |
29 (51.8%) |
67 (53.6%) |
n.s.† |
Obstetric DIC scores |
7.0 ± 3.3 |
6.6 ± 4.0 |
6.5 ± 3.0 |
n.s.* |
Blood loss [gram] |
1,352 ± 835 |
1,129 ± 658 |
1,254 ± 770 |
n.s.* |
IUFD |
16 (24.2%) |
11 (19.6%) |
30 (24.0%) |
n.s.† |
Delivery [weeks] |
29.8 ± 1.1 |
32.5 ± 0.5 |
35.0 ± 0.8 |
0.0001* |
Delivery route (CD/NVD/VD) |
62/4/0 |
52/4/0 |
113/10/2 |
n.s.† |
Birth weight [gram] |
1,290 ± 285 |
1,716 ± 314 |
2,203 ± 344 |
0.0001* |
Neonatal sex male |
33 (50.0%) |
30 (53.6%) |
72 (57.6%) |
n.s.† |
Umbilical arterial pH at birth |
7.14 ± 0.20 |
7.13 ± 0.21 |
7.17 ± 0.17 |
n.s.* |
Apgar score |
|
|
|
|
less than 7 at 1 minute |
54 (81.8%) |
38 (67.9%) |
76 (60.8%) |
0.012† |
less than 7 at 5 minutes |
35 (53.0%) |
23 (41.1%) |
55 (44.0%) |
n.s.† |
Neonatal outcome without IUFD |
|
|
|
|
Favorable |
40 (80.0%) |
41 (91.1%) |
89 (93.7%) |
0.035† |
Poor |
10 (20.0%) |
4 (8.9%) |
6 (6.3%) |
Values are shown as mean ± SD or the number with percentage. Statistical analysis was performed by analysis of variance with post hoc test* and chi-square test†.
Abbreviations, VPT: very preterm, PT: preterm, LPT: late preterm, n.s.: not significant, PIH: pregnancy-induced hypertension, IUFD: intrauterine fetal death, FHR: fetal heart rates, DIC: disseminated intravascular coagulation, CD: cesarean delivery, NVD: normal vaginal delivery, VD: vacuum delivery.
As shown in Table 2, multiple logistic regression analysis revealed that factors that affected the prognosis in 131 subjects excluding intrauterine deaths were umbilical arterial pH (p<0.0001; OR 0.17 [0.069-0.417] at ΔpH: 0.1) and tocolytic therapy (p<0.0132; OR 28.5 [2.014-403.9]). Smoking habit and Apgar score at 5 minutes <7 were not significant.
Table 2. Results of multiple logistic regression analysis for poor neonatal outcome.
Putative risk factors |
z value |
p value |
OR [95%CI] |
Umbilical arterial pH at birth (ΔpH=0.1) |
3.87 |
0.0001 |
0.17 [0.069, 0.417] |
Tocolysis before delivery |
2.48 |
0.0132 |
28.5 [2.014, 403.9] |
Smoking habit |
1.20 |
0.2298 |
3.16 [0.483, 20.78] |
Apgar score at 5 minutes less than 7 |
0.71 |
0.4783 |
1.91 [0.319, 11.51] |
Abbreviations, z value: significance of regression coefficient, OR: odds ratio, 95%CI: 95% confidence intervals.
In a previous report, we found that abnormal FHR monitoring findings were the most informative prognostic factors for predicting fetal acidemia among its symptoms and laboratory findings in cases of placental abruption. Persistent bradycardia and recurrent late deceleration, in particular, showed a high odds ratio (50.3 and 15.1, respectively) [3]. Abnormal FHR monitoring findings are clearly markers of fetal acidemia not just in cases of placental abruption. Therefore, normal management of pregnancy and delivery is important in cases of placental abruption, as well. However, the expectant mother does not receive medical attention in a hospital because placental abruption often occurs in preterm. This ends up adversely affecting the perinatal prognosis; therefore, a highly accurate predictor of onset is needed. Baumann et al. reported a very useful predictive formula for this 7, but further studies have not been conducted and it is not being used in general clinical settings. One reason why no accurate predictor of onset is being used is the fact that the etiology of placental abruption is unknown. Ananth et al. [10] has suggested two hypotheses for the etiology 4. Their suggestion is based on the finding that preterm placental abruption and full-term placental abruption are pathologically different. A report by Tikkanen et al. [14] supports this hypothesis. They conducted a large-scale retrospective study in which the fetal male/female sex ratio in cases of placental abruption that occurred at 37 weeks or later was 1.11, but it increased to 1.49 when it occurred at 28-31 weeks. Therefore, we surmise that it is highly likely that the underlying mechanism of full-term placental abruption and that of preterm placental abruption are different. Thus, it may be important to investigate preterm placental abruption and full-term placental abruption separately when establishing a strategy for placental abruption.
2021 Copyright OAT. All rights reserv
The most important finding from this investigation is that tocolytic therapy is a factor involved in exacerbation of the neonatal prognosis in preterm placental abruption. The use of tocolytic agents is a common form of tocolytic therapy in many countries including Japan. The choice of drug used for this purpose differs from country to country, but ritodrine hydrochloride and magnesium sulfate are used in Japan. Oxytocin receptor antagonists (e.g., atosiban), indomethacin, calcium channel blockers (e.g., nifedipin), and nitroglycerin are not used in Japan. We were not able to shed light on the mechanism by which tocolytic therapy affects onset of preterm placental abruption in the present study. We can point out three possible reasons. The first possibility is that premature labor induces placental abruption. The second possibility is that tocolytic therapy delays the diagnosis of placental abruption. The third possibility is that the tocolytic agent itself causes or exacerbates placental abruption. The primary cause of preterm premature rupture of membranes and preterm labor is intrauterine infection [15]. Likewise, chorioamnionitis is the cause of preterm placental abruption. In this case, placental abruption occurs when matrix metalloproteinase activity increases, causing alteration of adhesion factors. This mechanism supports the hypotheses of Ananth et al. [4]. Intrauterine infection produces prostaglandin and induces labor pain. Tocolytic agents are used to treat this. Consequently, it may be possible to assume that the patient is prone to severe placental abruption when labor pain is the cardinal symptom.
The three major symptoms of threatened premature labor, which are recognized as symptoms of premature labor, are atypical genital bleeding, labor pain, and cervix dilatation [16,17], but the first two are the same as the initial symptoms of placental abruption. Moreover, given the fact that premature labor is clinically encountered 10 times more frequently than placental abruption, clinicians sometimes diagnose the initial symptoms of placental abruption as threatened premature labor. The 2013 annual report of the no-fault compensation system in Japan includes a case in which the patient developed placental abruption during management of threatened premature labor, resulting in cerebral palsy, and it warns of the importance of differentiation of placental abruption and threatened premature labor [18]. Unfortunately, this warning does not include concrete examples of how to differentiate them. Shah et al. [19] recommend the need for FHR monitoring for at least 4 hours in cases of traumatic placental abruption even if the trauma to the mother is mild since labor pain is seen in 100% of cases in the first 4 hours after onset [19]. Likewise, the results of the present study might shed light on differentiation of threatened premature labor and placental abruption. In cases of preterm placental abruption, cesarean section is performed within 8-10 hours of the initial symptoms (Table 1). Therefore, observation of symptoms for 10 hours is important regardless of whether tocolytic therapy is performed when the symptoms of threatened premature labor are noted. It might be necessary to consider the cost-effectiveness with respect to whether to use consecutive or intermittent FHR monitoring during this time.
The tocolytic agents used during this study was ritodrine hydrochloride. Based on the results of this investigation, we inferred that the use of the tocolytic agents exacerbated the prognosis of placental abruption, but we could not determine whether repression of labor pain was the cause or the drug was the cause. It has been demonstrated in vitro that beta2-stimulants suppress contraction by acting on uterine smooth muscle. However, it is not known how they act on peripheral vessels. There are several interesting reports regarding this. Saller et al. [20] reported that use of tocolytic agents was effective for improving the neonatal prognosis in cases with bleeding in the third trimester [20]. A similar report was published by Towers et al. [21]. Their investigation included cases with placenta previa, suggesting that it differs from our investigation of placental abruption only. The results of their investigation showed that the use of tocolytic agents for placenta previa at least alleviated bleeding. Therefore, it is surmised that it is unlikely that ritodrine hydrochloride is the cause of placental abruption or the cause of a poor prognosis.
The biggest limitation of this study could be the fact that it is a retrospective study. Because it is a multicenter study, it is unclear whether the entry criteria were the same. That is to say, the subjects registered may not be uniform. Furthermore, we were not able to determine by what mechanism use of tocolytic agents caused poor neonatal outcomes in subjects who developed placental abruption. A large-scale investigation such as a nationwide study may need to be carried out. Moreover, appropriate evaluation of intrauterine infection that takes into account the timing of onset may be necessary.
In conclusion, the present study showed that use of tocolytic agents is a possible factor that causes poor neonatal outcomes in preterm placental abruption. We were not able to establish that intrauterine infection was a cause, but the present findings suggest that follow-up observation during the first 10 hours after onset may improve the neonatal prognosis.
This study was supported by a grant from the Ogyaa Donation Foundation of the Japan Association of Obstetricians and Gynecologists. We thank Mr Sugimoto for kindly providing analyses of the database. We wish to thank the institutions and representative physicians enrolled in the database for Perinatal Research Network in Japan, which include: Aichi Medical University: S Kinoshita; Akita University: A Sato; Asahi-chuo-Hospital: H Udagawa, A Kurihara; Asahikawa Medical University: K Nishino; Ashikaga Red Cross Hospital: Y Kasuga, T Hirao; Ehime Prefectural Central Hospital: K Noda; Fukuchiyama City Hospital: T Okuda; Fukuoka University: T Yoshisato; Fukushima Medical University: H Takahashi; Gifu University: H Toyoki; Haga Red Cross Hospital: A Ohkuchi; Hamamatsu University School of Medicine: K Suzuki; Hirosaki University: T Higuchi; Hiroshima City Hospital : O Ishida; Hiroshima General Hospital: Y Nakanishi; Hiroshima University: Y Mukai; Hokkaido University: S Yamada; Hyogo College of Medicine: T Takenobu; Hyogo Prefectural Kobe Children's Hospital: T Funakoshi; Japanese Red Cross Fukuoka Hospital: M Nishida; Japanese Red Cross Kyoto Daiichi Hospital: H Yamamoto; Jichi Medical University: S Matsubara, R Usui; Juntendo University Urayasu Hospital: K Yoshida, A Tajima;Kagawa University: H Tanaka; Kagoshima City Hospital : M Kamitomo;Kagosima University: Y Yonehara; Kameda Medical Center: M Suzuki, H Takaya; Kanagawa Children's Medical Center: H Ishikawa; Kanazawa Medical University: T Fujita; Kinki University: M Shiota, M Tsuritani; Kitasato University: S Kawano; Kobe University: K Tanimura; Kumamoto City Hospital: J Ishimatsu , K Aikou; Kurashiki Medical Center: F Yamazaki; Kurume University: D Hori, R Hayashi; Kyoto Prefectural University of Medicine: T Okubo, S Fujisawa; Kyoto University: J Hamanishi; Kyushu University: K Fukushima; Maternal & Child Health Center AIIKU HOSPITAL: T Adachi, Y Kawana; Mie University: T Sugiyama; Miyazaki University: S Furukawa; Nagasaki Municipal Hospital: K Kotera; Nagasaki University: S Yoshimura; Nagoya Daini Red Cross Hospital: N Kato; Nagoya University : T Kotani; Nara Medical University: T Sado; National Center for Child Health and Development: H Sagou, H Aoki; National Center for Global Health and Medicine: J Kakogawa; National Defense Medical College: Y Hasegawa; National Hospital Organization East Saga Hospital: M Nomiyama; National Hospital Organization Nagasaki Medical Center: I Yasuhi, M Fukuda;National Hospital Organization Nishisaitama Chuo National Hospital: A Yoshida; National Hospital Organization Okayama Medical Center: Y Tateishi; National Hospital Organization Takasaki General Medical Center: I Ito; National Hospital Organization Yokohama Medical Center: A Nakamura; Niigata University: T Serikawa; Nippon Medical School Tama Nagayama Hospital: I Kawabata; Nippon Medical School: M Satomi; Oita Prefectural Hospital: S Sato; Oita University: Y Nishida; Okayama University: T Segawa; Osaka City University: D Tachibana, M Tsukioka; Osaka Medical Center and Research Institute for Maternal and Child Health: N Mitsuda, A Sasahaea; Osaka University: S Fujita; Saga University: M Muro; SaiseikaiYokohamashi Tobu Hospital: Y Konishi, Y Sakakibara; Shiga University of Medical Science: T Ono; Shimane University: S Aoki; Shinshu University: N Kikuchi; Showa University : R Matsuoka; St. Marianna University School of Medicine, Yokohama City Seibu Hospital: J Saito,TNaKo; Takatsuki General Hospital: S Nakago; Teikyo University Hospital: T Ayabe, K Kido; The Japan Baptist Hospital: H Egawa, S Suzuki; The Jikei University: S Wada; The University of Tokyo: Y Kamei; Toho University Omori Medical Center: C Aoki; Tohoku University: J Sugawara; Tokai University: H Ishimoto, K Mituzuka; Tokyo Medical University Hachioji Medical Center: T Nohira; Tokyo Medical and Dental University,University Hospital of Medicine: Y Momohara; Tokyo Women's Medical University : Y Matsuda, Y Makino; Tottori University: T Harada; University of Occupational and Environmental Health, Japan: K Yoshimura; University of Toyama: S Saito, A Shiozaki; University of the Ryukyus: K Sakumoto; Wakayama Medical University: S Yagi; Yamagata University: S Tsutsumi; Yamaguchi Red Cross Hospital: H Takahashi; Yodogawa Christian Hospital: C Mikami; Yokohama City University Medical center: M Okuda; Yokohama Minami Kyosai Hospital: H Nagase; Yokohama Rosai Hospital: M Nakayama.
- Report of maternal death in Japan during 2010 and 2012. Japan Association of Obstetricians and Gynecologists.
- Furukawa S, Sameshima H, Ikenoue T, Ohashi M, Nagai Y (2011) Is the perinatal outcome of placental abruption modified by clinical presentation? J Pregnancy 2011: 659615. [Crossref]
- Matsuda Y, Ogawa M, Konno J, Mitani M, Matsui H (2013) Prediction of fetalacidemia in placental abruption. BMC Pregnancy Childbirth 13: 156. [Crossref]
- Ananth CV, Getahun D, Peltier MR, Smulian JC (2006) Placental abruption in term and preterm gestations: evidence for heterogeneity in clinical pathways. Obstet Gynecol 107: 785-792. [Crossref]
- Ananth CV, Oyelese Y, Prasad V, Getahun D, Smulian JC (2006) Evidence of placental abruption as a chronic process: associations with vaginal bleeding early in pregnancy and placental lesions. Eur J Obstet Gynecol Reprod Biol 128: 15-21. [Crossref]
- Räisänen S, Gissler M, Nielsen HS, Kramer MR, Williams MA, et al.(2013) Social disparity affects the incidence of placental abruption among multiparous but not nulliparous women: a register-based analysis of 1,162,126 singleton births. Eur J Obstet Gynecol Reprod Biol 171: 246-251.[Crossref]
- Baumann P, Blackwell SC, Schild C, Berry SM, Friedrich HJ (2000) Mathematic modeling to predict abruptio placentae. Am J Obstet Gynecol 183: 815-822. [Crossref]
- Steinborn A, Rebmann V, Scharf A, Sohn C, Grosse-Wilde H (2003) Placental abruption is associated with decreased maternal plasma levels of soluble HLA-G. J Clin Immunol 23: 307-314. [Crossref]
- Elliott JP, Gilpin B, Strong TH Jr, Finberg HJ (1998) Chronic abruption-oligohydramnios sequence. J Reprod Med 43: 418-422. [Crossref]
- Ananth CV, Kinzler WL (2010) Clinical features and diagnosis of placental abruption.
- [No authors listed] (2000) Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol 183: S1-1S22. [Crossref]
- Terao T, Maki M, Ikenoue T (1987) A prospective study in 38 patients with abruptio placentae of 70 cases complicated by DIC. Asia Oceania J Obstet Gynaecol 13: 1-13. [Crossref]
- Matsuda Y, Maeda T, Kouno S (2005) Fetal/neonatal outcome in abruptio placentae during preterm gestation. Semin Thromb Hemost 31: 327-333. [Crossref]
- Tikkanen M, Luukkaala T, Gissler M, Ritvanen A, Ylikorkala O, et al. (2013) Decreasing perinatal mortality in placental abruption. Acta Obstet Gynecol Scand 92: 298-305. [Crossref]
- Goldenberg RL, Hauth JC, Andrews WW (2000) Intrauterine infection and preterm delivery. N Engl J Med 342: 1500-1507. [Crossref]
- Cunningham FG, Leveno KJ, Bloom SL, Hauth JC, Rouse DJ, Spong CY, editors. Williams Obstetrics, 23rd ed. New York: McGraw-Hill Companies, Inc; 2009. 814-815.
- Lockwood CJ (2013) Overview of preterm labor and birth.
- (2013) The 2013 annual report of the no-fault compensation system in Japan.
- Shah AJ, Kilcline BA (2003) Trauma in pregnancy. Emerg Med Clin North Am 21: 615-629. [Crossref]
- Saller DN Jr, Nagey DA, Pupkin MJ, Crenshaw MC Jr (1990) Tocolysis in the management of third trimester bleeding. J Perinatol 10: 125-128. [Crossref]
- Towers CV, Pircon RA, Heppard M (1999) Istocolysis safe in the management of third-trimester bleeding? Am J Obstet Gynecol 180: 1572-1578. [Crossref]

