Objective: The intrauterine device (IUD) is an important long-acting reversible contraceptive method (LARC) which plays a major role in contraception. Expulsion and intolerance because of pain and/or bleeding are two factors which limit more widespread use. IUDs have to form some type of ‘anchor’, which must be well tolerated in order not to be expelled and not cause problems as might any other intrauterine object. We considered how an IUD might behave in comparison to a physiological or pathological intra-uterine body.
Methods: We reviewed historical and present day IUDs on a three point rating scale of i) flexibility ii) horizontal to vertical ratio and iii) percentage increase in size of the IUD over mean cavity measurements to determine an Anchor Index (AI) to separate the types of anchor methods the various devices employ.
Results: The AI generally varied from 3-7 in multiparous women signifying a marginally ‘physiological’ fit. The AI was mainly above 5 for most IUDs in nulliparous women indicating a largely non- physiological (‘pathological’ type) fit.
Conclusions: The structure, composition and design of most IUDs are still not optimal for the nulliparous and occasionally the multiparous endometrial cavity. IUD design should be such that they appear to the endometrial cavity to be a physiological rather than a pathological incumbent.
intrauterine device, anchor index, endometrial cavity, displacement, dislocation, expulsion, embedment
The intrauterine device (IUD) is an important long-acting reversible contraceptive method (LARC) which plays a major role in contraception [1]. In order to be used successfully a pharmacologically active IUD has to do only two things 1) stay in good position and 2) not give problems. From the perspective of uterine physiology and pathology these two things are potentially mutually exclusive. Unfortunately the IUD has a number of possible problems which prevent its more widespread use. These include producing pain, erratic and excessive bleeding, possible infection, failure to prevent pregnancy and expulsion from the uterine cavity [2]. The management of these problems is variable and dependant on circumstances, except in the case of complete or partial expulsion. A copper IUD (not a hormonal one) which is partially expelled (downwardly displaced) more than 2cm must be removed. A new device should be inserted or a different birth control method provided. A hormonal IUD can remain as long as it is not in the cervical canal. In order, not to be expelled an IUD must anchor in the endometrial cavity. Anchorage can mimic a ‘physiological’ or ‘pathological’ intra-cavitary body. ‘Physiological” implies that it is accommodated by and sits comfortably in the endometrial cavity. ‘Pathological’ implies that it does not fit the endometrial cavity properly and is partially embedded or attached to the wall of the uterus.
Intrauterine pathologies which remain in the uterine cavity are of two types:
- large intra-cavitary bodies which may or may not be adherent to the uterine walls ((small non-adherent intra-cavitary bodies are likely to be ejected) (type I));
- those bodies which remain tethered (anchored) to the wall by a strand(s) of tissue (type 2).
Large intra-cavitary bodies which are not attached may include loose fibroids or calcified cysts. Those pathologies which are attached only by a strand to the uterine wall include pedunculated fibroids, polyps, and certain carcinomata. The majority of these pathological conditions will produce pain which is related to the attempted expulsion of the intrauterine pathology i.e. it may be continuous [3]. The pain may later settle and recur only with menses as the dislodging of pathological tissue induces expulsive cramps.
Ideally then the design of an intrauterine device should attempt to promote it to behave as a ‘physiological’ foreign body which indefinitely fails to produce an expulsive trigger and thus remains retained and symptom free in the endometrial cavity. Non-ideally the IUD may behave as a pathological foreign body in the uterine cavity which the uterus is unable to eject, but in so doing produces symptoms (pain and/or bleeding). In this situation the IUD may well become asymptomatic after a few weeks and pain recurs with menses as additional blood and endometrial tissue enters the uterine cavity and acts as a ‘pathological trigger’ for repeated uterine cramps.
In the past, many IUD researchers have attempted to develop new IUD designs with the aim to reduce side-effects and maximise continuation of use (Figure 1). The purpose of this paper is to examine the type of anchor mechanisms some of these IUDs have used previously and currently and to see how closely they appear to have approximated to a ‘pathological’ type anchor, or a ‘physiological’ type anchor which has been designed to minimise problems. This paper examines how we can accommodate these two potentially mutually exclusive considerations.

Figure 1. Older IUDs, not in use anymore, and currently used IUDs . (Museum M. Thiery, Pand, Ghent, Belgium)
A review of the most prominently used IUDs for which adequate information concerning their size, type of material and insertion method was undertaken. The work began examining IUDs from the introduction by Richter in 1909 [4], and Pust in 1923 [5]. Data from living subjects was not involved and there was no requirement for ethical consideration for data but ethical approval and patient consent for reproduction of clinical graphics was obtained. Additionally patient identification was removed from clinical illustrations.
Using recent information on uterine cavity size and uterine physiological action an estimate of the degree of “physiological” versus “pathological” anchoring was attempted [6-21]. The evaluation included the type of material used in the manufacture of the IUD. Less pliant material would render an IUD less ‘physiological’. This is because a stiffer device would act more like a calcified intrauterine body than physiological intrauterine tissue which is more compliant since it is not normally calcified.
Secondly a horizontal to vertical ratio was determined as this attempts to predict how likely the IUD is to fit in the endometrial cavity which behaves as an isosceles trapezoid [11]. Finally the dimensions of the IUD were examined relative to the dimensions of the known average size of the multiparous and nulliparous endometrial cavity as determined by ultrasound, and other visual means [10].
These measurements were used to calculate a scoring system for each device which would indicate how likely the device was to anchor in a physiological or pathological manner. The index was derived by scoring each of these three features:
Flexibility: Flexibility decreased if the IUD is composed of non-flexible metal rather than plastic. Moulded polyethylene is more flexible than moulded polypropylene (no longer used except sometimes for threads), but is rendered less flexible if impregnated with barium. Flexible metal such as nitinol is now available. The total absence of a frame gives maximum flexibility. A scoring system in which a point is given for each feature which decreases flexibility was made on a three point rating scale with 1 being the most flexible and 3 being the least flexible (Table 1).
Horizontal to vertical ratio of the IUD: The smaller the horizontal to the vertical (H/V) ratio of an IUD the more likely it is to fit physiologically into the endometrial cavity [13]. This is in keeping with the shape of the endometrial cavity which is functionally an isosceles trapezoid. This feature is especially prominent in the nulliparous endometrial cavity which exhibits “high waisting” (due to elevated fundal myometrial promontories (Figure 2) and usually has a smaller H/V ratio than the multiparous uterus. This difference between the nulliparous and multiparous H/V ratio was not admitted into the present calculations for the sake of simplicity, and having to introduce another ratio. The data therefore tend to underestimate the size effect of most IUDs in the nulliparous endometrial cavity and lead to a lower pathological score in nulliparae than if these corrections had been made. A scoring system from 1-3 depending on this ratio was derived (Table 1).
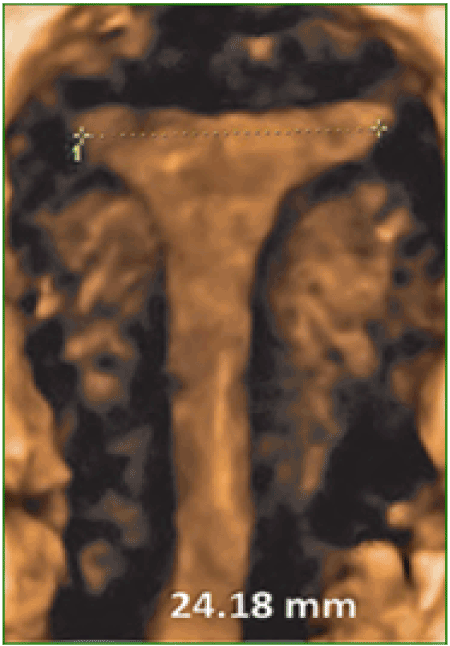
Figure 2. Example of a nulliparous uterus which exhibits ‘high waisting’ (age 18).
Percentage increase in the dimensions of the IUD: The percentage increase of either the vertical or the horizontal arm of an IUD over the mean ultrasonically/visually determined length and/or width of an endometrial cavity was also calculated on a three point scale (Table 1).
Table 1. Determination of pathological versus physiological Anchor Index(AI)† for IUDs.
- IUD flexibility
|
Maximal (1) |
Moderate (2) |
Minimal (3) |
- horizontal/vertical ratio(H/V)
|
0.6-0.7(1) |
0.8-0.9(2) |
0.9+(3) |
- % increase of length and/or width of IUD over the length or width of the endometrial cavity for multiparous and nulliparous uterus
|
nil-2.4%(1) |
2.5%-4.9%(2) |
5%+(3) |
Value in parentheses is the designated score for each parameter
†- AI is a+b+c
The value of each of these parameters was summed and the value obtained expressed as the Anchor Index (AI). The higher the anchor index the more likely the IUD will remain in the uterus by mimicking a pathological versus a physiological method. A summary of the data used to calculate the Anchor Index (AI) which indicates if an IUD anchors largely as a pathological body or is in at least modest harmony with the uterine cavity is given in Table 2. The index has been constructed so that the greater the AI, the more likely the particular IUD is anchoring using a quasi- pathological rather than a quasi-physiological method. Arbitrarily a cut-off point of 5 was chosen. Devices with an AI of 5 or greater are more likely to be anchoring pathologically than those with a score of 4 or less.
The IUDs which have been studied and for which an adequate amount of information is available are listed in Table 2. The table includes many older devices which are no longer in use. They are included so that it is possible to see the changes in development in the field and to see if the later and current methods differ from the earlier ones and whether current devices anchor in a similar or different manner. Using this information a determination was made to attempt to establish the most likely type of anchor for each device and how likely it is to act as a physiological or pathological anchor, depending on its AI (Table 3).
Table 2. Dimensions and composition of selected IUDs.
IUD |
Length (mm) |
Maximum horizontal width (mm) |
H/V |
Principal components |
Grafenberg ring |
25 |
25 |
1 |
copper, nickel, silver |
Lippes loop A |
26.2 |
22.2 |
0.85 |
Alathon-20 PE +BS |
Lippes loop B |
25.2 |
27.4 |
1.1 |
Alathon-20 PE +BS |
Lippes loop C,D |
27.5 |
30.0 |
1.1 |
Alathon-20 PE +BS |
Copper 7 (Gravigard) |
36 |
26 |
0.72 |
Polypropylene + BS |
Copper 7 mini |
28 |
22 |
0.78 |
Polypropylene + BS |
Progestasert |
36 |
32 |
0.89 |
PE+BS |
Copper T¶ |
36 |
32 |
0.89 |
PE + BS |
Multiload Cu (standard)¶ |
36 |
18 |
0.5 |
PE |
Multiload Cu short¶ |
25 |
18 |
0.72 |
PE |
Multiload Cu375SL¶ |
29 |
18 |
0.62 |
PE |
Multiload Cu 250 mini |
25 |
12 |
0.48 |
PE |
Nova T¶ |
32 |
32 |
1.0 |
PE+BS |
Skyla(Jaydess)¶ |
30 |
28 |
0.95 |
PE+BS |
Mirena¶ |
32 |
32 |
1.0 |
PE+BS |
Flexi-T300¶ |
28 |
23 |
0.82 |
PE + BS |
Flexi-T+¶ |
32 |
28 |
0.88 |
PE + BS |
Femilis 28 |
30 |
28 |
0.95 |
pEVA+ BS in cross-arms |
Femilis 24 |
30 |
24 |
0.8 |
pEVA+ BS in cross-arms |
GyneFix¶ |
20 |
2.2 |
0.05 |
copper on suture thread of polypropylene |
Fibroplant |
35.5 |
1.6 |
0.05 |
pEVA+BS |
IUBA¶ (intra-uterine ball) |
12 |
12 |
1.0 |
Copper balls on nitinol thread |
IUBB¶ |
15 |
15 |
1.0 |
as above |
IUBC¶ |
18 |
18 |
1.0 |
as above |
Veracept¶ |
32 |
30 |
0.94 |
T-shaped with copper at tips. Frame is nitinol |
Composition of the IUDs in this table were compiled from Population Reports, Series B, John’s Hopkins University Baltimore, MD, 1979-2006. Data from the manufacturers was used for the newer devices.
Abbreviations: PE-polyethylene, BS-barium sulphate, pEVA-polyethylene vinyl acetate
Lippes loop A-D, Ortho pharmaceuticals, Raritan, New Jersey, USA.
Copper-7 and mini, GD Searle, Skokie, Illinois, USA.
Prgestasert, Alza Corp, Palo Alto, California, USA.
Copper T 200(Ortho)and TCu380A /Paragard, Teva Pharmaceuticals, Pensylvania, USA.
Multiload Cu, Multilan, Fribourg, Switzerland.
Nova T, Bayer Pharmaceuticals, Wupprtal, Germany.
Skyla/Jaydess, Bayer Pharmaceuticals, Wupprtal, Germany.
Mirena, Bayer Pharmaceuticals, Wupprtal, Germany.
Flexi-T+ and 300, Prosan International BV, Arnhem, Netherlands.
Femilis24 and 28, Contrel Be, Ghent, Belgium.
GyneFix, Contrel Be, Ghent, Belgium.
Fibroplant, Contrel Be, Ghent, Belgium.
IUB A-C, Ocon Medical, Modiin, Israel.
Veracept, Contramed, Campbell, California, USA.
¶-indicates device is currently commercially available
The Lippes Loop A had an AI of 5 in nulliparae, and is no longer available [20]. The B, C and D versions (AI of 6,6 and 7 in multiparous) were intended for use by multiparous women only [22]. The Copper T with an AI of 7, and Nova T, and Mirena with an AI of 8 appear to anchor most pathologically in nulliparae. Skyla (Jaydess) and Femilis 28 with an AI of 7 appear marginally more adapted in nulliparae, but are still not ‘physiological’ in this group i.e meet the AI target of 5 or less. Of the newer IUDs, the Flexi-T and Femilis 24 have lower AI values (AI 5) and GyneFix has the lowest (AI 3), mimicking a physiological anchor in nulliparae. GyneFix is a special case and is discussed later. The newer IUDs with lower AIs also appear to anchor more physiologically in multiparae. The intrauterine ball(IUB) appears to have a more physiological fit in both nulliparous and multiparous users because of its extreme flexibility and small dimensions (AI 5) (Table 3). Its failure to achieve an AI of less than 5 is only because it is spherical and does not approximate well to the horizontal to vertical ratio of the endometrial cavity.
Table 3. Physiological versus pathological anchor scores and Anchor Index(AI) for selected IUDs.
IUD |
flexibility (a) |
H/V ratio (b) |
increase over uterine cavity length and/or width (multipa-rous)†(c) |
increase over uterine cavity length and/or width (nullipa-rous)†(d) |
AI multiparous a + b + c |
AI nulliparous a + b + d |
Expulsion rate |
comments |
Grafenberg ring/ Stainless Steel ring [13] |
3 |
3 |
1 |
1 |
7 |
7 |
16.3 (at 1 year) |
pathological retention |
Lippes loop A |
2 |
2 |
1 |
1 |
5 |
5 |
|
designed for nulliparous |
Lippes loop B |
2 |
3 |
1 |
N/A |
6 |
N/A |
|
Not optimal for nulliparous |
Lippes loop C |
2 |
3 |
1 |
N/A |
6 |
N/A |
|
|
Lippes loop D [15] |
2 |
3 |
2 |
N/A |
7 |
N/A |
5.2-13.0 (at 1year) |
designed for multiparous only |
Copper 7 (Gravigard) [13] |
3 |
2 |
1 |
3 |
6 |
8 |
12.8 (at 3 years) |
Not optimal for nulliparous |
Copper 7 mini |
3 |
1 |
1 |
1 |
5 |
5 |
|
designed for ‘small’ uterus |
Copper T |
2 |
2 |
2 |
3 |
6 |
7 |
|
pathological fit in nulliparous only |
Progestasert |
2 |
2 |
2 |
3 |
6 |
7 |
|
pathological fit in nulliparous only |
Multiload Cu 250 [13] |
1 |
1 |
1 |
1 |
3 |
3 |
7.4 (at 4 years) |
|
Multiload Cu short |
1 |
2 |
1 |
1 |
4 |
4 |
|
|
Multiload Cu375 SL [13] |
1 |
1 |
1 |
1 |
3 |
3 |
6.8 (at 4 years) |
|
Multiload Cu mini |
1 |
1 |
1 |
1 |
3 |
3 |
|
high expulsion rate |
Nova T [16] |
2 |
3 |
2 |
3 |
7 |
8 |
6.2 (at 5 years) |
pathological in nulliparous, borderline in multiparous |
Mirena [16,18] |
2 |
3 |
2 |
3 |
7 |
8 |
5.7-11.8 (at 5 years) |
pathological fit in nulliparous only, borderline in multiparous |
Skyla (Jaydess) [18] |
2 |
3 |
1 |
2 |
6 |
7 |
4.6 (at 3 years)‡ |
not optimal for nulliparous |
Femilis 28 [19] |
2 |
3 |
1 |
2 |
6 |
7 |
0.4 (at 5 years) |
not optimal for nulliparous |
Flexi T [26] |
2 |
2 |
1 |
1 |
5 |
5 |
6.8 (at 3 years) |
physiological in both groups |
Flexi T+ |
2 |
2 |
1 |
1 |
5 |
5 |
|
Not optimal for nulliparous |
Femilis 24 |
2 |
2 |
1 |
1 |
5 |
5 |
|
physiological in both groups |
GyneFix¶ [26] |
1 |
1 |
1 |
1 |
3 |
3 |
0.6-5.4 (at 5 years) |
most physiological cavity fit |
Fibroplant |
1 |
1 |
1 |
1 |
3 |
3 |
|
|
IUBA [21] |
1 |
3 |
1 |
1 |
5 |
5 |
|
|
IUBB [21] |
1 |
3 |
1 |
1 |
5 |
5 |
|
|
IUBC [21] |
1 |
3 |
1 |
1 |
5 |
5 |
|
|
Veracept [21] |
1 |
3 |
2 |
3 |
6 |
7 |
|
|
†multiparous endometrial cavity length 44mm, width 28mm (H/V=0.63)
‡Includes partial expulsion
†nulliparous endometrial cavity length 37mm, width 25mm (H/V=0.67)
¶Type II pathological suspension (deliberate). The term ‘Expulsion’ has a broader meaning when applied to the GyneFix as it includes failure to implant the knot in the fundal myometrium. Failure to implant the knot means that the device stays in the uterine cavity but not attached to the uterine wall as intended. This results in the expulsion of the frameless IUS within days or weeks of the attempted insertion.
N/A-not applicable
The first indication that uterine anatomy was taken into consideration when designing an IUD is from the publications of Lippes [22]. He used the measurements from Dickinson’s anatomical text book [23] to design the Lippes loops. Lippes was hampered by the problems that ‘inert’ (non-copper or hormonal) IUDs require a large surface area to be effective [24]. This limits the ability to make them to conform to uterine anatomy and physiology.
After the discovery of copper as an anti-fertility agent it became possible to drastically reduce the size of the frame of the IUD as the frame became the carrier of the anti-fertility agent and not the anti-fertility agent itself. It was Tatum who realized that a ‘T’ shaped IUD would have the ability, not only to have an anatomical fit to the endometrial cavity but a physiological one as well [25]. All this depended on the ‘T’ frame being the correct size. Unfortunately, the information available at the time, from Dickinson’s post-mortem studies and hysterography was not correct. With hindsight, we know that the measurements (especially the horizontal one) chosen by Tatum were too large. The ‘T’ shaped IUD is the device which is most likely to anchor physiologically if it is the correct size as the outer arms will sit on the myometrial promontories and the stem will remain in the endometrial cavity where it can resist expulsive forces as the device is in harmony with the endometrial cavity (Figure 3). A ‘T’ shaped device which does not fit the cavity precisely no longer enjoys the advantage of a physiological anchor but develops a pathological anchor as it embeds in the myometrial wall. This is the cause of many ‘T’ IUD related problems (Figure 4A-C).
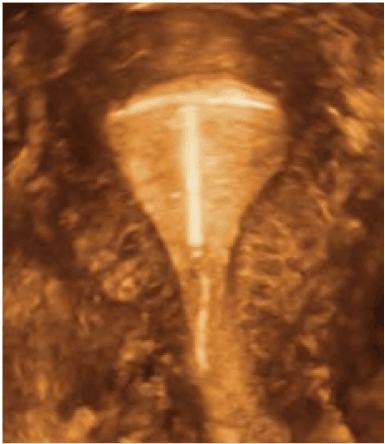
Figure 3. 3-D ultrasound picture of T-shaped IUD (Femilis) of which outer arms lean on the myometrial promontories will resist expulsive forces, as the device is in harmony with the uterine cavity (age 32).
Ideally, framed IUDs should fit the endometrial cavity with precision, above the promontory. In this case the likelihood of problems (e.g, expulsion, embedment) in any group, nulliparous or multiparous is drastically reduced [26]. If the transverse arm of the IUD is too long as in Figure 4A-C, then the IUD will either be expelled due to symmetrical expulsive forces or the anchor becomes pathological, causing embedment and bleeding due to asymmetrical uterine forces acting on the IUD [27]. In the latter case, the uterus may not be capable of expelling the IUD. Figure 5 shows an example of a severely distorted uterine cavity caused by spatial discrepancy between the IUD and the endometrial cavity.
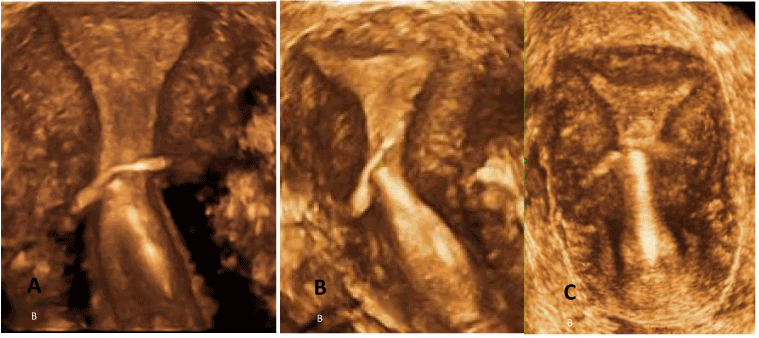
Figure 4. A-C. 3-D ultrasound illustrations of pathological situations as it embeds in the myometrial wall (TCu380A). Note the narrow fundal transverse diameter of the uterine cavity (age 27).
The AI is a very approximate method to predict the possible behaviour of an IUD as it is based on the mean uterine cavity width and length of parous and nulliparous women. The shorter the width and length of the endometrial cavity, the more likely a pathological type of anchoring is going to occur. We have deliberately made no attempt to try to correlate this value with event data from large multicentre clinical trials because of the heterogenous nature of subjects in these studies. In order to test this hypothesis a type of randomised crossover study design would be needed. For ethical reasons only subjects experiencing problems would be crossed over. There would be many ways to analyse the data but this is beyond the scope of the present study. A high AI which suggests pathological type anchoring does not appear to be directly related to expulsion as the IUD may become embedded instead of being expelled. Lower expulsion rates in nulliparous than in parous women seen in some clinical trials could simply be due to a higher rate of embedment of the IUD [28]. Side-effects i.e. cramping pain and abnormal bleeding do not however necessarily correlate with the high AI. There is too much anatomical and physiological uterine variation for this to occur. Benacerraf et al. [9] have shown that in symptomatic IUD users undergoing sonography for bleeding and/or pain the IUD was more likely to be mal-positioned. In addition, abnormally placed IUDs were more likely to be seen using 3D rather than 2D ultrasound. IUD expulsion in symptomatic IUD users may solve the woman’s problem of IUD intolerance while she is put at risk of unintended pregnancy. Conversely, if the IUD is not expelled, the endometrial trauma caused by embedment and distortion of the uterus will continue to result in abnormal bleeding and pain, leading to early request for removal of the IUD. In another study Van Schoubroeck et al found that in more than 50% of parous and nulliparous women apparent embedment was noted on 3-D ultrasonic measurements only 6 weeks after insertion of the IUD [29]. It could not be confirmed if the embedment represents the presence of a penetrating transverse arm or a true secondary perforation. Their observation that the presence of ‘embedding’ did not influence the pain score, does not rule out its factual or potential clinical importance. It rather illustrates that the simple visualization that the device itself was not of a size to fit ‘properly within the uterus’ to guarantee a long-term patient tolerability and functionality as LARC [29].
Whilst IUD design and size of the IUD are important to ensure a ‘harmonious co-habitation’ between the IUD and the host uterine cavity, the insertion procedure and type of the IUD is of major significance. T-shaped IUDs of which the transverse arms are folded upward in the inserter tube at the time of insertion, and those of which the arms remain outside the tube, or are folded downward in the tube, will behave totally differently when pushed out of the inserter tube. In both cases, too long transverse arms will likely embed in the wall, in the first case in the upper part of the uterus, especially since the arms are pushed against the (narrow) fundus as illustrated (Figure 5), whilst in the latter case in the mid or lower segment of the uterus (Figures 6 and 7), especially in case of IUDs with high H/V ratio (generally broader) as in the illustrations shown.
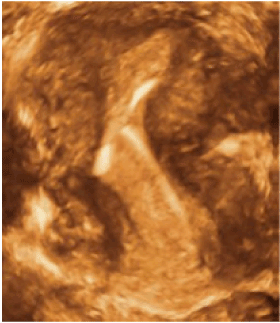
Figure 5. Severe distortion of the uterine cavity due to heavy uterine forces impacting on the IUD(TCu380A) (age 19).
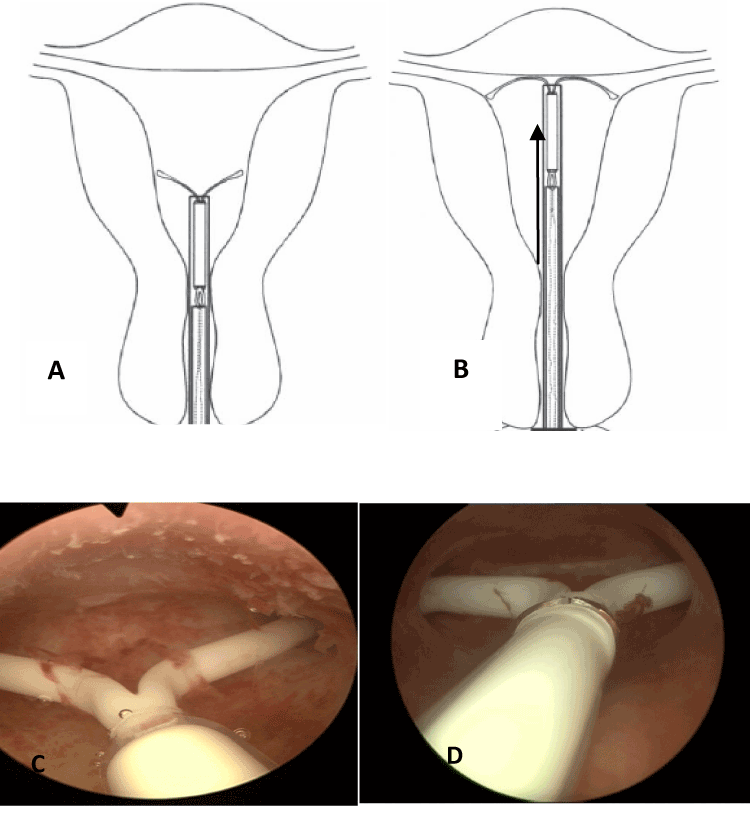
Figure 6. The insertion procedure of this framed device consists in pushing the released crossarms (Jaydess) with the inserter against the fundus of the uterus (A). If the arms are longer than the width of the uterine cavity, the tip of the arms may penetrate either the uterine wall (B) or the fallopian tubes (C), as shown in these illustrations(age 29).
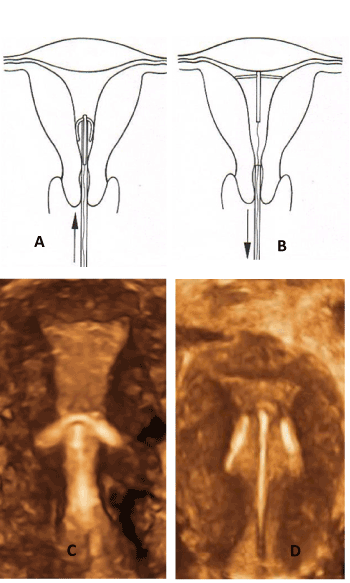
Figure 7. The insertion procedure differs completely from the one in Figure 6. The crossarms (Femilis) are pushed in the uterus and unfold when entering the cavity thereby protecting against perforation of the uterus (A). The transverse arm of the IUD should not be too long to allow proper positioning of the horizontal arm above the promontory. In young women, a retention arm of 24 or 28 mm is the correct length in most women. Pathologic anchoring (embedment) occurs due to a much too long transverse arm. These situations should not be allowed as they can cause harm and result mostly in early removal (age 19).
Logically it is better if IUDs mimic an intrauterine physiological entity rather than a pathological product. If that is the case we should expect the pain after insertion of a physiologically placed IUD to settle rapidly and there to be minimal exacerbation of pain with menses. Persistent pain which takes weeks to diminish and then recurs with menses is an indication that an IUD is not in properly adapted to the uterine cavity [30-33].
The frameless GyneFix IUD, from its material and size appears to be in perfect anatomical and physiological adaptation within the uterine cavity (Figure 8). However like the Multiload Cu250 mini (which had a very high expulsion rate) it lacks a mechanism for retention and so it is retained by suspension. This mimics the Type 2 pathological mechanism for retention of intrauterine bodies e.g. pedunculated polyps. It thus has the best of both situations, a physiological presence in the endometrial cavity which aids to tolerability, and a non-interfering pathological like suspensory mechanism to prevent expulsion.
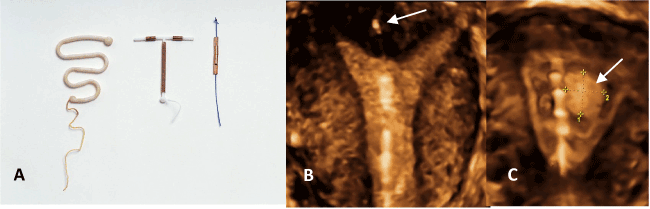
Figure 8. Lippes Loop, TCu380A and GyneFix 200 (A). The very low H/V ratio in the case of the frameless IUD enhances compatibility with most uteri. 3-D ultrasound of the frameless IUD illustrating physiological harmony with the uterine cavity. The anchor is visible in the fundus (arrow) (B). The frameless IUD adapts to the volume changes of the endometrial cavity despite the presence of a large space-occupying endometrial polyp (arrow) (age 35).
There are several limitations in this study. The size of the uterine (endometrial) cavity is very variable in both nulliparous and multiparous women [10]. The AI has been calculated based on the mean width and length of cavities in nulliparous and parous women. It is therefore impossible to determine the type of anchor in an individual user without having an indication about the width of the uterine cavity [26]. The width of the uterine cavity in 165 nulliparous women [10] had a mean width of 24.4 mm (range 13.8-35.0 mm),while 101 (62.7%) were below the 50th percentile which corresponds with studies conducted by others [7,9]. The many variations make it difficult for the practising gynaecologist to select the most suitable IUD as a standard size IUD may fit in one uterus but not in the other. However, proper fitting of IUDs is important as they will result in better performance and high continuation of use. The problems that may occur due to the lack of ‘physiological presence of the IUD cannot be corrected by counselling [34].
For practical purposes, as previously mentioned, the horizontal to vertical ratios of the IUDs in this study have not been adjusted for the sometimes marked differences in the nulliparous and multiparous endometrial cavity H/V ratios, and the marked difference of IUD performance in these groups [34]. This is common due to the ‘high waisting’ of the myometrial promontory (muscle on which the arms of an open IUD rest) of the nulliparous uterus (Figure 2) [35]. This has been considered in the development of some IUDs, most notably the frameless GyneFix copper IUD and the T-shaped Fermilis LNG-IUS [36,37]. This consideration would make some of the IUDs considered even more maladapted to the endometrial cavity than the H/V ratio values of 0.63 (parous women) and 0.67 (nulliparous women) which were used (Table 3).
An IUD which is in harmony with the uterine cavity should not produce pain for more than 3-7 days, not weeks after insertion, and it should not exacerbate menstrual pain. This is true for all physiologically retained intrauterine products until the moment of jettison i.e. cramps during menstruation or miscarriage and uterine labour activity during parturition. IUD pain which does not settle down rapidly may be anchoring in a similar manner to intrauterine pathological events.
All the IUDs which were evaluated and which anchor using a ‘pathological’ type method do so using the type I method i.e. acting as an overly large intracavitary body. These IUDs often lead to expulsion, embedment or early removal. Only the GyneFix deliberately uses the type 2 pathological anchor method, i.e. suspension from a uterine wall. Use of the type 2 method for the GyneFix is a deliberate use of ‘pathological’ type 2 anchoring unlike the unforeseen use of pathological anchoring for the devices using the type I pathological method. A newer approach is the use of very flexible frames eg the IUB and Veracept which use nitinol instead of polyethylene as a frame. Nitinol is more flexible and stronger than polyethylene so that the increased flexibility of the device is accompanied by a lower volume of material. Both these properties have theoretical advantages.
Currently used IUDs are generally not too long but they are usually too wide for the dimensions of the endometrial cavity [37]. The width of the cavity is simple to measure if proper equipment is available (3-D sonography is best performing) but is not practical in most clinical settings. Smaller IUDs which adapt to the narrow uterine cavity of nulliparous women are needed in order to improve performance and continuation of use [38]. A 24-mm transverse arm may be optimal for most of these women. Frameless IUDs fit in narrow as well as in large cavities. As the main reason for expulsion of IUDs (incompatibility) is not present with the frameless IUD, most expulsions should be regarded as failed insertions (failure to anchor the IUD properly in the fundus of the uterus). Therefore, expulsion has a different meaning when considering the frameless IUD as it is the intention to actually anchor the device to the fundus. A recent 2D and 3D ultrasound study has confirmed that the nulliparous endometrial cavity is too small for most framed IUDs [39].
In order to be well tolerated, IUDs should cause a minimum of distortion of the endometrial cavity during the maximum degree of the contraction phase [38]. The anchor mechanism should mimic the behaviour of a physiological intrauterine process rather than an intrauterine pathology. Many IUD providers still fail to understand the implications of an intrauterine device or system that is grossly in disharmony with the uterine cavity, leading to a quasi-pathological condition and its consequences.
Norman D Goldstuck: Nil
Dirk Wildemeersch: Dirk Wildemeersch has been involved in the optimization of new, innovative drug delivery systems for use in the uterus. He is currently an advisor in devising new concepts in controlled release for contraception, gynecological treatment, and prevention of infectious diseases for Contrel.
NG designed the study and researched the data and drafted the paper. DW helped analyse the data and helped write the paper and provided all illustrations. The authors read and agreed on the final manuscript. All illustrations belong to and are the copyright of DW. Clinical pictures are from his own and colleagues private practice.
2021 Copyright OAT. All rights reserv
This study was not financially supported.
For use of clinical pictures, and patient consent for graphics from GynMunster Outpatients and Endoscopic surgery, Munster, Germany, courtesy of Dr Thomas Hasskamp.
- Winner B, Peipert JF, Zhao Q, Buckel C, Madden T, et al. (2012) Effectiveness of long-acting reversible contraception. N Engl J Med 366: 1998-2007. [Crossref]
- Gimes D (2011) Intrauterine Devices. In: Hatcher RA, Trussell T, Nelson AL, Cates W, Kowal D, Policar MS, eds. Contraceptive Technology, 20th edition. New York, 511-544.
- Netter FH (1954) Diseases of the uterus. Reproductive System Volume 2, The Ciba collection of medical illustrations. Ciba Pharmaceutical Company, USA, 157-175.
- Richter R (1909) Ein Mittel zur Verhuetung de Konzeption. Deutsch Med Wschr 1909; 35: 1525-1527.
- Pust K (1923) Einbreich brauchbarer Frauenschutz. Deutsch Med Wschr 49: 952-953.
- Hasson HM (1984) Clinical studies of the Wing Sound II metrology device. In: Zatuchni GI, Goldsmith A, Sciarra JJ, editors. Intrauterine Contraception: Advances and Future Prospects. Philadelphia, PA, USA: Harper and Row 126-141.
- Kurz KH (1984) Cavimeter uterine measurements and IUD clinical correlation. In: Zatuchni GI, Goldsmith A, Sciarra JJ, editors. Intrauterine Contraception: Advances and Future Prospects. Philadelphia, PA, USA: Harper and Row 142-162.
- Kamal I, Hefnawi F, Ghonheim M, Talant M, Abdalla M (1971) Dimensional and architectural disproportion between the intrauterine device and the uterine cavity: a cause of bleeding. Fertil Steril 22: 514-521. [Crossref]
- Benacerraf BR, Shipp TD, Lyons JG, Bromley B (2010) Width of the normal uterine cavity in premenopausal women and effect of parity. Obstet Gynecol 116: 305-310. [Crossref]
- Kaislasuo J, Heikinheimo O, Lähteenmäki P, Suhonen S (2014) Predicting painful or difficult intrauterine device insertion in nulligravid women. Obstet Gynecol 124: 345-353. [Crossref]
- Hasson HM, Dershin H (1981) Assessment of uterine shape by geometric means. Contracept Deliv Syst 2: 59-75. [Crossref]
- Goldstuck N (2012) Assessment of Uterine Cavity Size and Shape: A Systematic Review Addressing Relevance to Intrauterine Procedures and Events. Afr J Reprod Health 16: 129-138. [Crossref]
- Goldstuck ND (1982) The relationship of IUD dimensions to event rates. Contracept Deliv Syst 3: 103-105. [Crossref]
- Rowe PJ (1994) Clinical performance of copper IUDs. In: Bardin WC, Mishell D, editors. Proceedings from the Fourth International Conference on IUDs. Butterworth-Heinemann. Boston, USA.
- Cole LP, Edelman DA (1980) A comparison of the Lippes Loop and two copper-bearing intrauterine devices. Int J Gynaecol Obstet 18: 35-39. [Crossref]
- Andersson K, Odlind V, Rybo G (1994) Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use: a randomized comparative trial. Contraception 49: 56-72. [Crossref]
- Sivin I, el Mahgoub S, McCarthy T, Mishell DR Jr, Shoupe D, et al. (1990) Long-term contraception with the levonorgestrel 20 mcg/day (LNg 20) and the copper T 380Ag intrauterine devices: a five-year randomized study. Contraception 42: 361-378. [Crossref]
- Nelson A, Apter D, Hauck B, Schmelter T, Rybowski S, Rosen K, Gemzell-Danielsson K (2013) Two Low-Dose Levonorgestrel Intrauterine Contraceptive Systems – A Randomized Controlled Trial. Obstet Gynecol 122: 1205-1213. [Crossref]
- Wildemeersch D, Janssens D, Andrade A (2009) The Femilis LNG-IUS: contraceptive performance-an interim analysis. Eur J Contracept Reprod Health Care 14: 103-110. [Crossref]
- Van Kets HE, Van der Pas H, Delbarge W, Thiery M (1995) A randomized comparative study of the TCu380A and Cu-Safe 300 IUDs. Adv Contracept 11: 123-129. [Crossref]
- Hsia JK, Creinin MD (2016) Intrauterine Contraception. Semin Reprod Med 34: 175-182. [Crossref]
- Lippes J (1989) The Making of the First Loop. In van der Pas MFM, Dieben TOM, eds. State of the art of the IUD. Dordrecht, The Netherlands: Kluwer Academic Publishers 12-20.
- Dickinson RL (1971) Human Sex Anatomy. Robert E Krieger Publishing Co., Huntington, New York.
- Moyer DL, Mishell DR (1971) Reactions of Human Endometrium to the Intrauterine Foreign body II. Long term effects on the endometrial histology and cytology. Am J Obstet Gynecol 111: 66-80. [Crossref]
- Tatum NJ (1989) The Conception, Incubation, Infancy, Adolescence and Maturity of the ‘T’. In Van der Pas HFM, Dieben Th OM, eds. State of the art of the IUD. Dordrecht, the Netherlands: Kluwer Academic Publishers 24-26.
- Wildermeersch D, Pitt A, Jandi S, Hasskamp T, Rowe P, et al. (2013) Precision Intrauterine Contraception May Significantly Increase Continuation of Use: A Review of Long-Term Clinical Experience with Frameless Copper-Releasing Intrauterine Contraception Devices. Int J Women’s Health 5: 215-225. [Crossref]
- Goldstuck ND, Wildemeersch D (2014) Role of uterine forces in intrauterine device embedment, perforation, and expulsion. Int J Womens Health 6: 735-744. [Crossref]
- Madden T, McNicholas C, Zhao Q, Secura GM, Eisenberg DL, et al. (2014) Association of age and parity with intrauterine device expulsion. Obstet Gynecol 124: 718-726. [Crossref]
- Van Schoubroeck D, Van den Bosch T, Ameye L, Veldman J, Hindrycks A, et al. (2013) Pain and bleeding pattern related to levonorgestrel intrauterine system (LNG-IUS) insertion. Eur J Obstet Gynecol and Reprod Biol 171: 154-156. [Crossref]
- Berger GS, Edelman DA, Regenie SJ (1976) Patient's responses to IUD insertion. Int J Gynaecol Obstet 14: 147-152. [Crossref]
- Ylikorkala O, Kauppila A, Siljander M (1978) Anti-prostglandin therapy in prevention of side-effects of intrauterine contraceptive devices. Lancet 2: 393-395. [Crossref]
- Goldstuck ND (1981) Pain response following insertion of a Gravigard (Copper 7) Interauterine Contraceptive Device in Nulliparous Women. Int J Fertil 26: 53-56. [Crossref]
- Goldstuck ND (1981) A comparison of the initial pain response following insertion of the Copper 7 and combined Multiload Copper 250-short IUDs. Contracept Deliv Syst 2: 295-301. [Crossref]
- Aoun J, Dines VA, Stovall DW, Mete M, Nelson CB, et al. (2014) Effects of age, parity, and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol 123: 585-592. [Crossref]
- Goldstuck ND (1984) The combined Multiload Copper 250 Short IUD In Women With Reduced Uterine Cavity Measurements. In Bonnar J, Thompson W, Morrison RF, eds. Research in Family Planning. Lancaster, United Kingdom: MTP Press Limited 125-128.
- Wildemeersch D, Janssens D, Andrade A (2009) The Femilis LNG-IUS: contraceptive performance-an interim analysis. Eur J Contracept Reprod Health Care 14: 103-110. [Crossref]
- Wildemeersch D, Jandi S, Pett A, Nolte K, Hasskamp T, et al. (2014) Use of frameless intrauterine devices and systems in young nulliparous and adolescent women: results of a multicenter study. Int J Womens Health 6: 727-734. [Crossref]
- Tatum HJ, Connell EB (1989) Intrauterine devices. In: Filshie M, Guillebaud J, editors. Contraception: Science and Practice. London, UK: Butterworths.
- Widemeersch D, Hasskamp T, Nolte K, Jandi S, Pett A, et al. (2016) A multicenter study assessing uterine cavity width in over 400 nulliparous women seeking IUD insertion using 2D and 3D sonography. Eur J Obstet Gynecol 206: 232-238. [Crossref]








