Abstract
Pulmonary Veno-occlusive Disease (PVOD) is a rare cause of pulmonary hypertension with high morbidity and mortality. Impressive clinical signs and symptoms often obscure the true underlying capillary disorder, thus severely compromising timely and appropriately directed therapy. The hemodynamics of PVOD is the consequence of a widespread vascular obstructive process that originates in the pulmonary venules and small veins. Without curative lung or heart-lung transplantation, patients with this condition face inexorable clinical deterioration and death within months to a few short years of initial presentation. We report a case of 19-year old male who presented with progressive shortness of breath, fatigue and hypoxemia. Clinical examination was compatible with right ventricular failure and investigations including transthoracic echocardiogram and right heart catheterization confirmed presents of pulmonary arterial hypertension. Extensive work-up for secondary causes was negative. Thoracic CT scan findings were consistent with diagnosis of veno-occlusive disease supported by reduced CO diffusion capacity and normal ventilation perfusion lung scan. He was treated with diuretics, oxygen and pulmonary vasodilator therapy which he tolerated well without developing pulmonary edema but in view of no significant clinical improvement and PVOD being incurable, he was referred for lung transplant.
Key Words
pulmonary hypertension, veno-occlusive disease, computed tomography, ventilation perfusion lung scan, transthoracic echocardiogram, right heart catheterization, lung transplant
Case Report
An 18-year-old male was referred from a local hospital with history of progressive shortness of breath for six months. He also complained of a syncopal attack on exertion lasting for few seconds. The patient had no significant past medical history except mild intermittent bronchial asthma controlled on inhaled albuterol as required. He denied use of any medication and there was no family history of respiratory or cardiac illness. On examination, he was found to have regular heart rate of 90 beats per minute, respiratory rate of 22 breaths per minute, blood pressure of 117/74 mmHg and an oxygen saturation of 91% on room air. Cardiopulmonary examination revealed raised jugular venous pressure, positive left parasternal heave, loud pulmonary component of 2nd heart sound, tricuspid regurgitation murmur, minimal basal crackled on chest auscultation and mild pedal edema. Abdominal examination showed soft, non-tender, lax abdomen with no organ enlargement and neurological examination was normal.
Laboratory including complete blood count, liver and kidney functions were within normal range. Pro-B type natriuretic peptide was elevated at 654 pg/mL (normal <100 pg/mL). Screening for autoantibodies was negative.
Chest X-ray (Figure 1) revealed enlarged pulmonary arteries and increased bronchovascular markings. ECG showed sinus tachycardia, right axis deviation and prominent P wave.
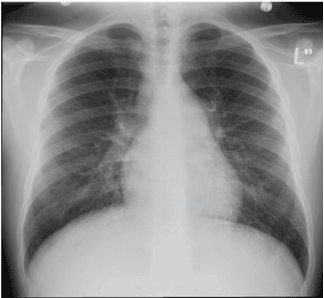
Figure 1. Chest X-ray showing enlarged pulmonary arteries and increased bronchovascular markings
A 2-dimensional transthoracic cardiac echocardiogram showed a severely dilated right ventricle, moderate tricuspid regurgitation with estimated systolic pulmonary artery pressure of 50 mmHg. Left ventricle was normal with preserved systolic function. Right heart catheterization result revealed mean pulmonary artery pressure >60 mmHg, Right atrial pressure 6 mmHg, pulmonary capillary wedge pressure 8 mmHg, pulmonary vascular resistance 13.78 Wood units and cardiac index 1.98 L/m/min.
A computed tomographic angiogram (CTA) of chest (Figure 2) did not show any evidence of pulmonary embolism but there were dilated main pulmonary arteries, enlarged right atrium and ventricle, septal thickening with nodularity, centrilobular ground glass densities and moderate sized hilar and mediastinal lymphadenopathy highly suggestive of pulmonary veno-occlusive disease. In view of patient’s critical condition and typical clinical and imaging features, lung biopsy was not done.
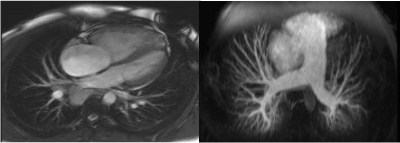
Figure 2. Computed tomographic angiogram (CTA) of chest showing enlarged pulmonary arteries, dilated right atrium and ventricle and bilateral septal thickening with nodularity and centrilobular ground glass densities and moderate sized hilar and mediastinal lymphadenopathy.
Pulmonary function tests showed mild restrictive pattern and significantly reduced CO diffusion capacity (DLCO). Cardiac magnetic resonance imaging (MRI) (Figure 3) showed dilated pulmonary artery with diameter of 3.9 cm, D-shaped left ventricle with normal ejection fraction, severe right ventricular dilatation with impaired function and no evidence of intra-cardiac shunt. Ventilation – perfusion lung scan was normal (Figure 4).
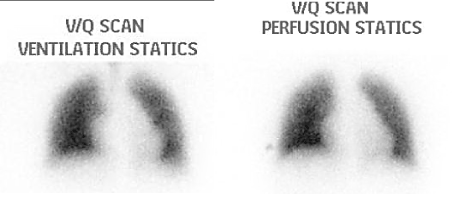
Figure 3. Cardiac magnetic resonance imaging (MRI) showing dilated pulmonary artery with diameter of 3.9 cm, D-shaped left ventricle and severe right ventricular dilatation.
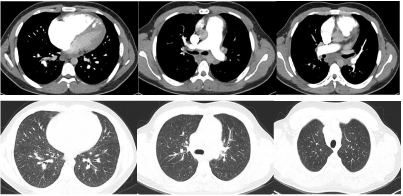
Figure 4. Ventilation Perfusion Lung Scan showing ventilation and perfusion distribution with no evidence of mismatch.
In view of typical thoracic CT scan features, normal ventilation-perfusion lung scan, reduce DLCO and severe pulmonary hypertension, patient was labeled as having group 1 pulmonary arterial hypertension due to idiopathic veno- occlusive disease. He was started on diuretics and oxygen. Pulmonary vasodilator therapy was initiated under closed supervision in the form of Sildenafil which he tolerated well and later Endothelin receptor antagonist Bosentan was initiated. Patient did shown improvement in 6-minute walk and oxygen requirement but continues to have functional class III. He was referred for lung transplant.
Discussion
Pulmonary veno-occlusive disease (PVOD) is a rare and incurable disorder that presents with features similar to pulmonary arterial hypertension (PAH). It is caused by obliterative fibrotic venopathy, predominantly affecting the smaller branches of the pulmonary venous tree.
PVOD along with pulmonary capillary haemangiomatosis (PCH), is placed in a separate but related group to PAH, referred to as Group 1’ in the current classification of pulmonary hyeptension [1]. The estimated annual incidence of PVOD is 0.1-0.2 cases per million in general population [2]. It is difficult to discern the incidence and prevalence accurately as many cases of PVOD are likely misclassified as idiopathic pulmonary hypertension. Some reports suggest that PVOD accounts for 5-20% of cases classified as idiopathic pulmonary arterial hypertension. PVOD can affect a wide spectrum of ages ranging from 9 days to 70 years old. However, it is mainly reported in children and young adults [2,3]. PVOD does not have a gender predilection for patients <20 years old, however, men are affected twice as frequently as women in older age group [4-7]. PVOD was first described in 1934 [8] but was accepted as a separate clinical entity in 1960 [9]. PVOD can be idiopathic or associated with number of conditions. An immune-mediated cause has been suggested due to the sporadic occurrence of PVOD in patients with underlying systemic lupus erythematosus, scleroderma, systemic sclerosis, human immunodeficiency virus infection, rheumatoid arthritis, Hashimoto thyroiditis and Raynaud phenomenon. Infectious etiology has been proposed in several patients with PVOD who developed a recent viral illness [10-12]. Serological evidence suggestive of recent Toxoplasma gondii and measles infection has been reported in some patients with PVOD [13-17]. Genetic risk factors have been proposed due to the development of several cases of PVOD in siblings [18-20]. PVOD has also been reported in cancer patients following radiation and chemotherapy and after peripheral blood stem cell and bone marrow transplantation [21,22]. Overall, many of the etiological questions remain unanswered. Dutch pathologist C.A.Wagenvoort proposed that PVOD is an acquired syndrome consisting of venous thrombotic occlusion induced by multiple insults to the venous endothelium [23]. Due to clinical symptoms and signs that often resemble and impersonate other disorders, timely and appropriately directed therapy may be compromised. It has been debated whether PVOD and Pulmonary Capillary Hemangiomatosis (PCH), a disorder affecting the pulmonary capillaries rather than the venules, are two distinct diseases or are varied expressions of a single disorder [24].
Hemodynamically, PVOD presents as pulmonary arterial hypertension with elevated pulmonary artery pressure and a normal or low pulmonary capillary wedge pressure (PCWP) on right-sided heart catheterization. The normal to low PCWP is theorized to be due to the fact that PVOD affects the venules and small veins variably which allows some fraction of collateral bronchial veins and spared patent venous tributaries to provide perceived normal venous outflow into the larger veins and left atrium during PCWP measurement. PVOD hemodynamics also results in increased capillary hydrostatic pressure resulting in transudation of fluid and the development of pulmonary edema [25].
Clinically, PVOD presents as a clinical triad consisting of severe PAH, radiographic evidence of pulmonary edema and a normal pulmonary artery occlusion pressure. Radiologically, PVOD depicts dilated central pulmonary arteries with thickened interlobular septa. The hemodynamic manifestations of PVOD are also reflected on imaging, with findings of septal lines, ground-glass opacities and pleural effusion. In a study of eight patients with PVOD it was observed that 50% or more of CT examinations demonstrated septal and fissural thickening, enlarged pulmonary arteries, normal pulmonary veins and bilateral pleural effusions [26]. Resten et al. identified septal lines and centrilobular ground-glass opacities as the two most helpful CT findings that help distinguish PVOD from Primary Pulmonary Hypertension [27,28]. Ventilation-perfusion scans obtained in a patient with PVOD is usually normal but can be misdiagnosed as chronic thromboembolic disease [29,30].
PVOD can only be diagnosed definitively by surgical lung biopsy but because of the high surgical risk in the presence of severe pulmonary hypertension, biopsy is not recommended and diagnosis can be made based upon clinical and specific radiological features in the presence of normal left heart [31,32]. PVOD is unique in a way that patients started on pulmonary vasodilator treatment may suffer an acute and potentially life-threatening deterioration and pulmonary edema [33]. The prognosis of PVOD is poor, with most reported patients dying within 2 years [34]. Pulmonary vasodilator therapies do not appear to change the survival in patients with PVOD.
Pharmacological management of PVOD is only a bridge towards the more definitive treatment which is lung or heart-lung transplantation.
Due to the rarity of PVOD, randomized clinical trials have not been feasible to see the efficacy and safety of pharmacologic agents in treating patients with PVOD. Most of the data regarding treatment is only available in case reports and case series. Hemodynamically, the goal of conventional medical therapy is to decrease pulmonary vascular resistance, increase cardiac output and reduce volume overload. No consensus is Selective pulmonary vasodilators typically used in PAH like oral phosphodiesterase-5 inhibitors and endothelin receptor antagonists have been shown to improve some patients both hemodynamically and clinically, however, clinical experience has shown that potent vasodilators including intravenous prostacyclin and calcium channel blockers may induce florid and even fatal pulmonary edema in patients in patients with PVOD [35,36]. Lung transplantation remains the only treatment that can prolong the lives of PVOD patients. The utility of this treatment is limited by the fact that the average waiting times for lung transplantation exceed the life expectancy of patients with PVOD. In patients who undergo successful lung transplantation, recurrence has not been reported except in one case [37].
Since the prognosis of PVOD patients is very poor, it is recommended to discuss lung transplantation option early in the course of disease. Further research is needed to understand the pathophysiological mechanism of this very rare form of pulmonary hypertension and to develop therapeutic strategies to improve survival.
References
2021 Copyright OAT. All rights reserv
- Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, et al. (2015) ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 46: 903-975.
- Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, et al. (2006) Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 173: 1023-1030. [Crossref]
- Holcomb BW Jr, Loyd JE, Ely EW, Johnson J, Robbins IM (2000) Pulmonary veno-occlusive disease: a case series and new observations. Chest 118: 1671-1679. [Crossref]
- Wagenvoort CA, Wagenvoort N, Takahashi T (1985) Pulmonary veno-occlusive disease: involvement of pulmonary arteries and review of the literature. Hum Pathol 16: 1033-1041. [Crossref]
- Nawaz S, Dobersen MJ, Blount SG Jr, Firminger HI, Petty TL (1990) Florid pulmonary veno-occlusive disease. Chest 98: 1037-1039. [Crossref]
- Cohn RC, Wong R, Spohn WA, Komer M (1991) Death due to diffuse alveolar hemorrhage in a child with pulmonary veno-occlusive disease. Chest 100: 1456-1458. [Crossref]
- Justo RN, Dare AJ, Whight CM, Radford DJ (1993) Pulmonary veno-occlusive disease: diagnosis during life in four patients. Arch Dis Child 68: 97-100. [Crossref]
- Hora J (1934) Zur histologie der klinischen ‘primaren pulmonalsklerose’. Frankf Z Pathol 47: 100.
- Heath D, Segel N, Bishop J (1966) Pulmonary veno-occlusive disease. Circulation 34: 242-248. [Crossref]
- Veeraraghavan S, Koss MN, Sharma OP (1999) Pulmonary veno-occlusive disease. Curr Opin Pulm Med 5: 310-313. [Crossref]
- Leinonen H, Pohjola-Sintonen S, Krogerus L (1987) Pulmonary veno-occlusive disease. Acta Med Scand 221: 307-310. [Crossref]
- Johnson SR, Patsios D, Hwang DM, Granton JT (2006) Pulmonary veno-occlusive disease and scleroderma associated pulmonary hypertension. J Rheumatol 33: 2347-2350. [Crossref]
- Brown CH, Harrison CV (1966) Pulmonary veno-occlusive disease. Lancet 2: 61-65. [Crossref]
- Heath D, Segel N, Bishop J (1966) Pulmonary veno-occlusive disease. Circulation 34: 242-248. [Crossref]
- Stovin PG, Mitchinson MJ (1965) Pulmonary hypertension due to obstruction of the intrapulmonary veins. Thorax 20: 106-113. [Crossref]
- Rosenthal A, Vawter G, Wagenvoort CA (1973) Intrapulmonary veno-occlusive disease. Am J Cardiol 31: 78-83. [Crossref]
- Bjornsson J, Edwards WD (1985) Primary pulmonary hypertension: a histopathologic study of 80 cases. Mayo Clin Proc 60: 16-25. [Crossref]
- Davies P, Reid L (1982) Pulmonary veno-occlusive disease in siblings: case reports and morphometric study. Hum Pathol 13: 911-915. [Crossref]
- Voordes CG, Kuipers JR, Elema JD (1977) Familial pulmonary veno-occlusive disease: a case report. Thorax 32: 763-766. [Crossref]
- Lucas RV Jr (1972) Congenital causes of pulmonary venous obstruction. Cardiovasc Clin 4: 19-51. [Crossref]
- Ibrahim NB, Burnley H, Gaber KA, Ormerod LJ, Addis B, et al. (2005) Segmental pulmonary veno-occlusive disease secondary to lung cancer. J Clin Pathol 58: 434-436. [Crossref]
- Salzman D, Adkins DR, Craig F, Freytes C, Le-Maistre CF (1996) Malignancy-associated pulmonary veno-occlusive disease: report of a case following autologous bone marrow transplantation and review. Bone Marrow Transplant 18: 755–760. [Crossref]
- Wagenvoort CA, Wagenvoort N, Takahashi T (1985) Pulmonary veno-occlusive disease: involvement of pulmonary arteries and review of the literature. Hum Pathol 16: 1033-1041. [Crossref]
- Wagenvoort CA, Beetstra A, Spijker J (1978) Capillary haemangiomatosis of the lungs. Histopathology 2: 401-406. [Crossref]
- Rambihar VS, Fallen EL, Cairns JA (1979) Pulmonary veno-occlusive disease: antemortem diagnosis from roentgenographic and hemodynamic findings. Can Med Assoc J 120: 1519-22. [Crossref]
- Swensen SJ, Tashjian JH, Myers JL, Engeler CE, Patz EF, et al. (1996) Pulmonary venoocclusive disease: CT findings in eight patients. AJR Am J Roentgenol 167: 937-940. [Crossref]
- Resten A, Maitre S, Humbert M, Rabiller A, Sitbon O, et al. (2004) Pulmonary hypertension: CT of the chest in pulmonary venoocclusive disease. AJR Am J Roentgenol 183: 65-70. [Crossref]
- Shackelford GD, Sacks EJ, Mullins JD, McAlister WH (1977) Pulmonary venoocclusive disease: case report and review of the literature. AJR Am J Roentgenol 128: 643-648. [Crossref]
- Rich S, Pietra GG, Kieras K, Hart K, Brundage BH (1986) Primary pulmonary hypertension: radiographic and scintigraphic patterns of histologic subtypes. Ann Intern Med 105: 499e502. [Crossref]
- Bailey CL, Channick RN, Auger WR, Fedullo PF, Kerr KM, et al. (2000) "High probability" perfusion lung scans in pulmonary venoocclusive disease. Am J Respir Crit Care Med 162: 1974-1978. [Crossref]
- Montani D, Price LC, Dorfmuller P, Achouh L, Jaïs X, et al. (2009) Pulmonary veno-occlusive disease. Eur Respir J 33: 189-200. [Crossref]
- Palmer SM, Robinson LJ, Wang A, Gossage JR, Bashore T, et al. (1998) Massive pulmonary edema and death after prostacyclin infusion in a patient with pulmonary veno-occlusive disease. Chest 113: 237e40. [Crossref]
- Davis LL, deBoisblanc BP, Glynn CE, Ramirez C, Summer WR (1995) Effect of prostacyclin on microvascular pressures in a patient with pulmonary veno-occlusive disease. Chest 108: 1754-1756. [Crossref]
- De Vries TW, Weening JJ, Roorda RJ (1991) Pulmonary veno-occlusive disease: a case report and a review of therapeutic possibilities. Eur Respir J 4: 1029-1032. [Crossref]
- Kuroda T, Hirota H, Masaki M, Sugiyama S, Oshima Y, et al. (2006) Sildenafil as adjunct therapy to high-dose epoprostenol in a patient with pulmonary veno-occlusive disease. Heart Lung Circ 15: 139-142. [Crossref]
- GugnaniMK, Pierson C, Vanderheide R, Girgis RE (2000) Pulmonary edema complicating prostacyclin therapy in pulmonary hypertension associated with scleroderma: a case of pulmonary capillary hemangiomatosis. Arthritis Rheum 43: 699–703. [Crossref]
- Izbicki G, Shitrit D, Schechtman I, Bendayan D, Fink G, et al. (2005) Recurrence of pulmonary veno-occlusive disease after heart-lung transplantation. J Heart Lung Transplant 24: 635-637. [Crossref]




