cerebral misery perfusion, cognition, stroke, revascularisation, carotid occlusion
A 51- yr old Caucasian man who ran a successful plumbing business was admitted with sudden left hemiparesis and confusion. MRI confirmed an acute right frontal lobe cerebral infarction. Neuropsychological assessment revealed impaired visual perception and construction, and left visual neglect. There was impairment with sequencing of tasks, working memory, and higher executive function. His hemiparesis resolved quickly but the cognitive deficits subsequently resulted in loss of his business and a massive financial debt accumulation.
Ultrasound, MR angiogram and cerebral angiography showed bilateral proximal carotid artery occlusions and established external to internal flow but with poor run off. Single Photon Emission CT (SPECT) with acetazolamide (Figure 1) confirmed significant hypoperfusion with little reserve in right middle cerebral and both anterior cerebral territories. A diagnosis of Stage 3 cerebral misery perfusion syndrome was made (Table 1). Two and a half months after the index stroke the patient underwent right pterional craniotomy and extracranial-intracranial(EC-IC) high saphenous bypass (Figure 2). Post-operatively he demonstrated a remarkable improvement in cognition. In the patient’s own words, it was as if ‘a cloud had been lifted from my mind’. His family noticed an improvement in speech, memory and even in his handwriting. An MMSE performed on the day before surgery was 13/30 and 4 days later was 30. An improvement was noted in all the domains of cognition. On review at 6 months, 1 year and 2 years the improvement in all domains of physical ability and cognition was sustained. He repaid his financial loans and restarted a successful business. His social skills, memory, interactions with family and colleagues, became normal once again. The patient and his family were extremely satisfied with the results of the surgery.
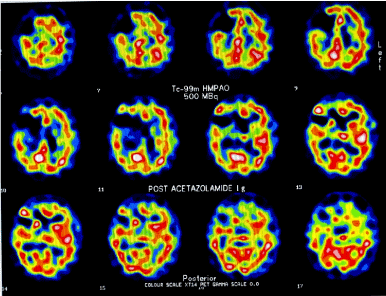
Figure 1. . Images from patient with cerebral misery perfusion syndrome: SPECT images showing decreased perfusion in right cerebral hemisphere following acetazolamide challenge./p>
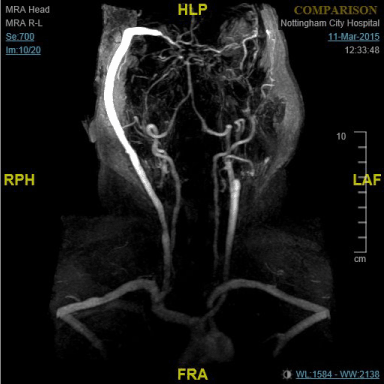
Figure 2. MR angiogram showing high-flow saphenous bypass graft connecting to branch of middle cerebral artery.
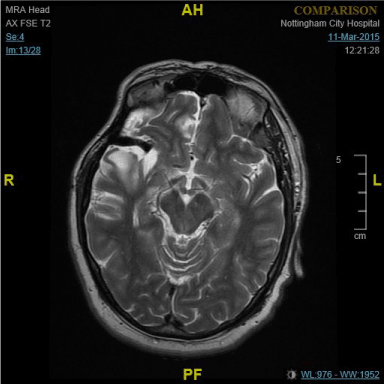
Figure 3. MRI Brain showing old Right frontal infarction and flow-void of EC-IC bypass graft.
Table 1. Pathophysiology of cerebral misery perfusion (Stage 1 to 3 cerebral haemodynamic impairment) [3].
Stage 1: Cerebrovascular autoregulation |
Any fall in regional cerebral perfusion pressure (rCPP) is matched
by a fall in regional cerebrovascular resistance (rCBR) in order to
maintain regional cerebral blood flow (rCBF). This is accommodated
by vasodilatation and an attendant increase in regional cerebral
blood volume (rCBV). Oxygen extraction factor (OEF) remains
constant. |
Stage 2: Misery perfusion |
The capacity for compensatory vasodilatation is exceeded (rCVR
becomes a constant) and rCBF therefore drops in tandem with rCPP.
To meet their metabolic demands, neurones must "extract more
oxygen" from the passing blood - OEF increases. |
Stage 3: End-organ compromise |
If rCBF continues to fall to the extent that the brain can no longer
compensate by increases in OEF, end-organ dysfunction occurs
(TIA). If this situation persists, permanent end-organ damage
(stroke) occurs. |
Carotid occlusion is not an uncommon sonographic or angiographic finding in symptomatic and asymptomatic patients undergoing workup for TIA or stroke. Almost a third to half of these patients have dysregulation with their cerebral circulation [1]. Baron coined the term ‘Cerebral misery perfusion’ (CMP) in 1981 to describe this failure of cerebral autoregulation. CMP is associated with decreased cerebral perfusion pressures in complete carotid artery occlusion, extracranial and intracranial atheromatous diseases, and Moyamoya disease [2].
We have previously shown that simple but unique methods can be used to diagnose CMP [3]. PET scan is the gold standard but is expensive and provides absolute measurements for both the oxygen extraction fraction (OEF) and Cerebral blood flow (CBF); it is also cumbersome and not widely available. Other modalities, such as the BOLD-contrast fMRI , SPECT, Xenon-enhanced CT, Dynamic contrast enhanced CT, MRI perfusion imaging and arterial spin labeling MR techniques are newer, promising and less invasive techniques to assess rCBF [4,5].
Focal ischaemic deficits can occur with carotid occlusion resulting in cerebral misery perfusion (amaurosis fugax , limb shaking [6], and retinal claudication [7]). Carotid occlusions are often asymptomatic and detected incidentally on the wrong side during investigation of TIA or stroke [8]. Sometimes chronic ocular ischaemia presenting as a ‘venous stasis retinopathy’ [9] and recurrent syncopal episodes [10] have also been reported.
Surgical revascularisation was proposed as a possibility for preventing cognitive impairment as far back as 1950 [11]. The temporal relation between the surgical revascularisation and the improvement in cognitive function in our patient clearly demonstrates the effectiveness of the procedure in patients with cerebral misery perfusion. The beneficial effect remained sustained in our patient for over 2 years with a successful return to work.
Does carotid occlusion lead to global ischaemic dysfunction following CMP? It is conceivable that when a large part of the brain loses its blood supply, some degree of cognitive impairment will ensue. Cognitive dysfunction and frank dementia as a true complication of this condition have been described in case reports [12]. A Norwegian study demonstrated cognitive impairment in individuals with asymptomatic carotid stenosis none of whom had suffered a stroke. A dose-response relationship was observed between degree of carotid stenosis and some of the cognitive tests, which can be considered a real argument for real associations [13]. What is interesting is that many of these patients who did not have any visible structural MRI lesions, still had poorer performance on neuropsychological tests. In the ENOS trial patients with ischaemic stroke secondary to ipsilateral carotid occlusion were found to have worse cognitive impairment at 90 days post stroke than those with no occlusion, following adjustment for age, sex and baseline stroke severity. When considered as a binary variable, 50% of patients with occlusion were cognitively impaired (MMSE-t<20) versus 33% with no occlusion [14].
We believe that patient selection and surgical expertise are crucial determinants of the outcome and previous trials may have suffered as a result of these two outcome- limiting factors. Patient selection has to be dictated by neuroimaging criteria, specifically perfusion asymmetry.
In the original EC/IC bypass study, STA-MCA anastomosis with primary end-points of 30-day stroke mortality and stroke incidence, recruitment of patients was done without reference to cerebral perfusion studies. Hence it was impossible to conduct subgroup analyses in patients with radiologically confirmed CMP [15,16]. Improvement in cognition after EC-IC bypass surgery has been reported by some [12] but not by others [17,18].
Reliance on the superficial temporal artery (STA) as the donor vessels have largely met with mixed results [16] .While surgical expertise was demonstrated with high graft patency rates, exceeding 90%, it is an important point of our case report that donor STA vessel may provide an inadequate blood flow rate.
Revascularisation utilising the saphenous vein graft can provide an immediate blood flow in excess of 140- ml per minute and this compares to flow in STA of 28- ml per minute. In experienced hands revascularisation utilising the saphenous vein can be performed with relatively low morbidity and mortality and provides immediate augmentation of CBF. Long term graft patency rates are acceptable and there are minimal atherosclerotic changes in the vein. In our patient CTA performed one year post-operatively confirmed that the graft was patent. CTP combined with CTA has been shown to be a safe and efficient modality as a follow-up evaluation tool for both hemodynamic change and bypass patency after STA-MCA bypass in the chronic cerebral ischemic diseases [19].
It is therefore of specific note in this case that the patient self- reported an immediate remarkable improvement in cognition, and this was confirmed on serial neuro-psychological testing. He scored 30/30 on a Montreal cognitive assessments(MOCA) and on MMSE when seen 22 months after his surgery.
We believe more work is needed to see if revascularisation of selected patients with occluded carotid arteries and cerebral misery perfusion prevents not only strokes but also future cognitive impairment, and the relative benefit of high-flow versus low-flow grafts needs further study.
- Cerebral misery perfusion(CMP) is an important differential diagnosis in patients presenting with symptomatic steno-occlusive carotid disease.
- Cognitive impairment and strokes are important sequelae of CMP.
- Sophisticated investigations are required to diagnose CMP syndrome in patients with carotid occlusion.
- Carotid revascularisation is a possible therapeutic intervention for prevention or reversal of cognitive impairment and dementia in CMP.
- Larger studies are needed to identify CMP subgroups that may benefit from EC-IC bypass or endovascular revascularisation.
- The relative benefit of high-flow over low-flow bypass needs further trials.
2021 Copyright OAT. All rights reserv
- Gibbs JM, Wise RJ, Thomas DJ, Mansfield AO, Russell RW (1987) Cerebral haemodynamic changes after extracranial-intracranial bypass surgery. J Neurol Neurosurg Psychiatry 50: 140-150. [Crossref]
- Baron JC, Bousser MG, Rey A, Guillard A, Comar D, Castaigne P (1981) Reversal of focal "misery-perfusion syndrome" by extra-intracranial arterial bypass in hemodynamic cerebral ischemia: a case study with 15O positron emission tomography. Stroke 12: 454-459. [Crossref]
- Gordon AL, Goode S, D'Souza O, Auer AO, Munshi SK (2010) Cerebral misery perfusion diagnosed using hypercapnic blood-oxygenation-level-dependent contrast functional magnetic resonance imaging. J Med Case Reports 4: 54.
- Wintermark M, Sesay M, Barbier E, Borbély K, Dillon WP, Eastwood JD, et al. (2005) Comparative overview of brain perfusion imaging techniques. Stroke 36: 2032-2033. [Crossref]
- van Laar PJ, van der Grond J, Mali WP, Hendrikse J (2006) Magnetic resonance evaluation of the cerebral circulation in obstructive arterial disease. Cerebrovasc Dis 21: 297-306. [Crossref]
- Knoflach M, Matosevic B, Meinhart M, Rücker M, Furtner M, Zangerle A, et al. (2011) Prognostic relevance of limb shaking in symptomatic carotid artery occlusion. Cerebrovasc Dis 32: 35-40. [Crossref]
- Furlan AJ, Whisnant JP, Kearns TP (1979) Unilateral visual loss in bright light: an unusual symptom of carotid artery occlusive disease. Arch Neurol 36: 675-676. [Crossref]
- Thanvi B, Robinson TG (2007) Complete occlusion of extracranial internal carotid artery: clinical features, pathophysiology, diagnosis and management. Postgrad Med J 83: 95-99. [Crossref]
- Klijn CJ, Kappelle LJ, van Schooneveld MJ, Hoppenreijs VP, Algra A, Tulleken CA, et al. (2002) Venous stasis retinopathy in symptomatic carotid artery occlusion: prevalence, cause, and outcome. Stroke 33: 695-701. [Crossref]
- Kashiwazaki D, Kuroda S, Terasaka S, Ishikawa T, Shichinohe H, Aoyama T, et al. (2005) Carotid occlusive disease presenting with loss of consciousness. No Shinkei Geka 33: 29-34. [Crossref]
- Fisher CM (1951) Senile dementia- A new explanation for its causation. Can Med Assoc J 65: 1-7.
- Tatemichi TK, Desmond DW, Prohovnik I, Eidelberg D (1995) Dementia associated with bilateral carotid occlusions: neuropsychological and haemodynamic course after extracranial to intracranial bypass surgery. J Neurol Neurosurg Psychiatry 58: 633-636. [Crossref]
- Mathiesen EB, Waterloo K, Joakimsen O, Bakke SJ, Jacobsen EA, Bønaa KH (2004) Reduced neuropsychological test performance in asymptomatic carotid stenosis: The Tromso Study. Neurology 62: 695-701. [Crossref]
- The ENOS Trial Investigators (2006) Glyceryl trinitrate vs. control, and continuing vs. stopping temporarily prior antihypertensive therapy, in acute stroke: rationale and design of the Efficacy of Nitric Oxide in Stroke (ENOS) trial (ISRCTN99414122). Int J of Stroke 1: 245-249. [Crossref]
- Derdeyn CP, Cross DT 3rd, Moran CJ, Dacey RG Jr (2001) Reversal of focal misery perfusion after intracranial angioplasty: case report. Neurosurgery 48: 436-440. [Crossref]
- (1985) The EC/IC Bypass Study Group: Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. N Engl J Med 313: 1191-1200. [Crossref]
- Younkin D, Hungerbuhler JP, O'Connor M, Goldberg H, Burke A, Kushner M, et al. (1985) Superficial temporal-middle cerebral artery anastomosis: effects on vascular, neurologic, and neuropsychological functions. Neurology 35: 462-469. [Crossref]
- Nielsen H, Højer-Pedersen E, Gulliksen G, Haase J, Enevoldsen E (1986) Reversible ischemic neurological deficit and minor strokes before and after EC/IC bypass surgery. A neuropsychological study. Acta Neurol Scand 71: 317-320. [Crossref]
- Park JC, Kim JE, Kang HS, Sohn CS, Lee DS, Oh CW, et al. (2010) CT perfusion with angiography as a substitute for both conventional digital subtraction angiography and acetazolamide-challenged SPECT in the follow-up of post bypass patients. Cerebrovasc Dis 30: 547-555.



