Abstract
In this study, patches of glycerol preserved bovine pericardium (GBP) and tunica vaginalis (GTV) were used as implants for the reconstruction of large hernia in 22 animals (6 calves, 5 buffalo calves, 3 sheep, 2 goats, 2 donkeys and 4 foals) to improve the tensile strength and to stimulate the fibroplasia at the reconstruction sites. In each case the defect was repaired with GBP or GTV by either inlay or underlay techniques of implantation. All the cases were successfully treated and no major complications were observed after six months of surgical operation. The results of the present study indicated effective use of glycerolized bovine pericardium and tunica vaginalis for prosthetic hernioplasty. To our knowledge this investigation could be considered the first report on the use of tunica vaginalis as xenografts for hernioplasty in clinical cases of farm animals.
Key words
Abdominal wall defect; bovine pericardium; farm animals; inlay; tunica vaginalis; underlay.
Introduction
Reconstruction of large abdominal hernias remain a significant problem and a challenge to surgeons due to insufficient autogenously tissue for adequate abdominal wall closure with recurrence being a common outcome and hence, it is inevitable to avoid after the use of biomaterials [1- 4]. The use of biomaterial for repairing of abdominal wall defects is gaining increasing recognition to achieve a tension-free repair and has resulted in a significant reduction in postoperative pain, length of recovery period and the number of recurrence [5].
Repairing of large abdominal wall defects using prosthetic materials in animals is a good option but the optimal biomaterials should possess adequate strength, biocompatible and lack the development of hypersensitivity reactions [1].
The Bovine pericardium and tunica vaginalis showed several advantages over polypropylene mesh as they were inexpensive, easy to obtain and of having adequate dimensions that are sufficient for reconstruction of large sized abdominal defect [6]. Also, the higher elasticity of bovine pericardium made the site of implantation felt more pliable [7,8].
Despite the availability of the GBP and GTV, little attention has been paid to their use in reconstructive surgery of abdominal wall defects in clinical cases. Therefore, the aim of this study was to evaluate the effectiveness of using glycerolized bovine pericardium and tunica vaginalis for repair of the external abdominal wall hernias in clinical cases using inlay and underlay techniques to improve the tensile strength and to stimulate the fibroplasia at their construction site.
Materials and methods
Twenty-two animals (6 calves, 5 buffalo calves, 3 sheep, 2 goats, 2 donkeys and 4 foals) of 4-9months age were examined and surgically treated because of large abdominal wall hernias either at the umbilicus or ventro-lateral abdominal wall was admitted to the Mansoura Veterinary Teaching Hospital during the period of this study (September 2006 to August, 2008).
Collection of Tunica Vaginalis Sacs and Bovine Pericardium
Fresh bovine parietal tunica vaginalis sacs and pericardia were collected from the slaughterhouse from BSE free cattle with average age 1.5 - 2years immediately after slaughtering. The tunica vaginalis sacs were cut through a longitudinal incision parallel to the epidydimal attachment to the testis and in both materials the external loose connective tissue and fascia were stripped mechanically.
Preparation of Glycerolized Bovine Pericardium and Tunica Vaginalis
The sacs and pericardia were shaken serially in sterile normal saline for 60 minutes, transferred to a sterile glass bottle containing 50% sterile glycerol under aseptic condition and kept there for 3 hours. These pieces were then transferred to a second bottle containing 70% sterile glycerol for 3 hours at room temperature and were finally plugged into 99.5% sterile glycerol and stored at 4oC before implantation [9].
Anesthetic considerations
In ruminants, atropine sulphate 0.05 mg per kg body weight (ADWIA, Cairo, Egypt) and xylazine HCl 0.05- 0.1 mg/ kg body weight (Xylaject- ADWIA, Cairo, Egypt) premedication was used, then circular infiltration analgesia was applied using lidocaine Hcl 2%. General anesthesia was induced and maintained in foals and donkeys with a combination of Xylazine 0.5 mg/ kg body weight and Ketamine HCl 2.2 mg/kg body weight (Tekam50, HIKMA Pharmaceuticals, and Amman, Jordan) intravenously via jugular vein catheter. The affected animals were controlled either in lateral or dorsal recumbency opposite of the abdominal wall defect.
Surgical Techniques for Repair
The aim of surgery was to reduce the contents and close the abdominal defect so that herniation cannot recur. The prosthetic materials used for repair of hernias were GBP that used in 12 animals (3 calves 2 buffalo calves, 2sheep, 2 goats, a donkey,2 foals). GTV were applied in 10 animals (2 calves, 3 buffalo calves, 2 sheep, 3 goats, 1mares, 1 donkey, 1 foal) (Table 1). The sites of operation were prepared for aseptic surgery. The prosthetic material was chosen and prepared according to the size of the hernial ring. An elliptical skin incision was performed. The fibrous adhesions were dissected and the devitalized tissues were removed to expose the hernial ring. Following proper hemostasis, the hernial sacs were incised along one side of the hernial ring margin to create a fascial flap and a single layer of the used prosthesis were implanted using one of the following techniques:
Table 1. Prosthetic materials and techniques used for repair of umbilical and abdominal hernias in clinical cases.
Animal species |
Calves |
Buffalo
calves |
Sheep |
Goats |
Foals |
Donkeys |
Total |
Technique |
IL |
UL |
IL |
UL |
IL |
UL |
IL |
UL |
IL |
UL |
IL |
UL |
Pericardium |
2 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
_ |
1 |
12 |
Tunica vaginalis |
1 |
2 |
2 |
1 |
_ |
1 |
_ |
_ |
_ |
2 |
1 |
_ |
10 |
Total |
3 |
3 |
3 |
2 |
1 |
2 |
1 |
1 |
1 |
3 |
1 |
1 |
22 |
IL: Inlay technique
UL: underlay technique
a- Inlay technique
This technique was applied on 12 animals (Table 1). The prosthetic materials were fashioned to a suitable size to the hernial ring, then placed where their visceral sides facing the abdominal cavity. The fibrosed edges of the ring were refreshed and the patches were sutured to the ring by No.1 Polypropylene monofilament by simple continuous suture that interrupted only at the corners and blocked every three or four stitches (Figures 1, 2).
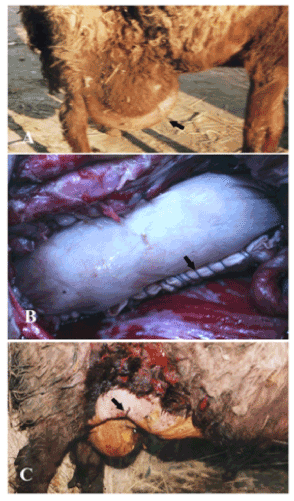
Figure 1. A case of ventro- lateral abdominal hernia in sheep (A) repaired by bovine pericardium using inlay technique (B) and after the operation (C).
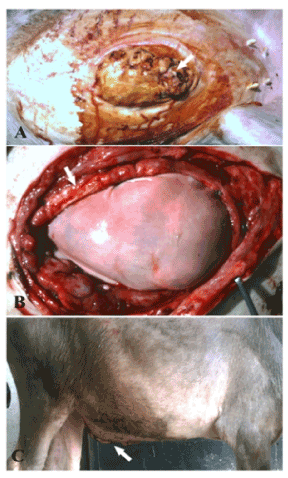
Figure 2. A case of infected umbilical hernia in buffalo calf (A) repaired by bovine pericardium using underlay technique (B) and after the operation (C).
b- Underlay technique
This technique was applied to 10 animals (3 calves, 2 buffalo calves, a sheep, a donkey and 3 foal) as shown in table 1. The prosthetic material was fashioned to the shape of the defect allowing for 4-5mm underlay then placed under the visceral peritoneum after omentalization and secured to the recipient tissue with interrupted overlapped pattern using No. 1 Polypropylene monofilament suture material (Figures 3, 4).
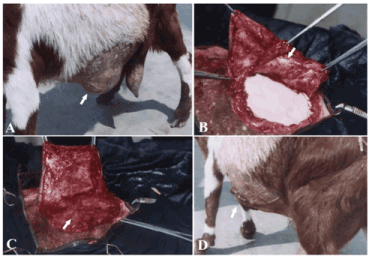
Figure 3. A case of ventro- lateral abdominal hernia in goat (A) repaired by tunica vaginalisusing inlay technique (B) and covered by a fascial flap derived from the hernial sac (C) and after the operation (D).
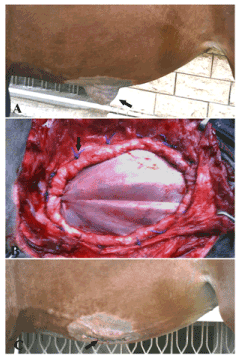
Figure 4. A case of ventral abdominal hernia in a filly foal (A) repaired by tunica vaginalisusing underlay technique (B) and after the operation (C)
The implants were covered with a fascial flap derived from the hernial sac when possible and the skin was sutured with horizontal mattress suture using silk No. 1. Cloth made abdominal belt was applied to all animal immediately after recovery from anesthesia and remained during the first postoperative week for protection. The operated animals were observed for six months after operation for presence of postoperative complications.
Postoperative care
Penicillin G procaine (Norocillin L.A, Norbrook Lab. limited, Newry, Ireland) and Flunixin meglumine (Flamicure, Pharma Swede, 10th of Ramadan, Egypt by 2.2 mg/kg) were injected for 5days post-surgery. The skin wound was dressed routinely with povidone iodine (Betadine-topical antiseptic). The operated animals were followed either by regular visits or calling the owners.
Results and discussion
On the basis of the available results, umbilical hernias were over presented than abdominal ones and were recorded in 13 animals (6 calves, 5 buffalo calves, 2 foals). The average diameter of the hernial ring was about 6x10 cms except in a buffalo calf where the hernia was associated with multiple subcutaneous sinuses discharging pus and the ring was 12cm in diameter and the hernial sac was adhered to the abomasums. Calves were commonly involved with a sex predilection to males [10,11]. In contrast to Mosbah and Karrouf [12] observations who reported the high incidence of umbilical hernias in females. Large hernias require safe closure because they interfere with athletic activities or normal parturition and they are cosmetically unsightly [13]. The successful repair of an abdominal wall defect is based on a tension-free closure to allow wound repair, a better collagen restoration and prevention of recurrence.
Ventral abdominal hernias in different animal species were also represented in this study as shown in Table 2. Sheep showed a high prevalence than other species with a high incidence in females [14]. Trauma constitutes the main cause of the abdominal hernia in the present study. Additionally, ventral abdominal hernia was recorded in sheep and goat following parturition; this may be due to abdominal distension caused by repeated pregnancies in such species and to the violent straining during parturition [15,16] who reported the frequent occurrence of ventral abdominal hernias in sheep and goat.
Table 2. Descriptive details of abdominal hernias in different animals.
Case no. |
Animal species |
Sex |
Hernia origin and location |
Character of hernial ring |
Origin |
Location |
Size(cms) |
Shape |
1 |
Ewe |
Female |
Recurrent |
Behind the right costal arch |
10x15 |
Circular |
2 |
Ewe |
Female |
Following parturition |
At level of the right stifle fold |
10x12 |
Oval |
3 |
Ewe |
Female |
Traumatic |
At prepubic region |
10x15 |
Circular |
4 |
Goat |
Female |
Following parturition |
At the left ventral abdominal wall |
8x10 |
Oval |
5 |
Goat |
Female |
Traumatic |
At level of the right stifle fold |
10x15 |
Circular |
6 |
Donkey |
Male |
Traumatic |
At level of the left stifle fold |
10x15 |
Circular |
7 |
Donkey |
Male |
Recurrent |
At level of the right stifle fold |
10x12 |
Oval |
8 |
Foal |
Female |
Traumatic |
At the left ventral abdominal wall |
10 x15 |
Oval |
9 |
Foal |
Female |
Traumatic |
At level of the right stifle fold |
10x12 |
Oval |
The good results achieved with the different prosthesis available in the present study and the reduction of the recurrence rate after the repair of these defects has put the prosthetic materials at the forefront with respect to the conventional suture repair techniques (Kingsnorth, 2000).
The fibrosed edges of the hernial rings were refreshed before implantation of the prosthetic materials in order to facilitate a better mesh incorporation with the host tissue [13]. Covering of the implanted materials with a supportive flap from the hernial sac was found to strengthen the abdominal wall closure and reduces postoperative complications attributed to excessive suture tension and seroma formation. Similar advice was given by [17] in repairing of incisional hernia in horses.
Although hernia recurrence was recorded in a goat implanted by using inlay technique following parturition this may be due to abdominal distension caused by pregnancy and to the violent straining during parturition despite the use of two folds of ovine pericardium which lead to recurrent herniation at the same site of the previously implanted patches [18]. Surprisingly, GTV gave a very good result after implantation in 10 animals. Skin wound infection was recorded only in 3 animals and disappeared following the use of local antiseptic; this could be attributed to contamination of the wound due to unhygienic condition during housing. No hernia recurrence was recorded during the follow up period of 6 months and the skin wound was healed completely within 3 weeks.
The glycerol pretreatment seems to be simple and inexpensive, delay implant biodegradation and replacement by host tissue compared with other types of preservation as lyophilized, irradiated freeze drying method [9,19]. While [6] added that the use of glutaraldehyde for the preservation of bovine pericardium originated from its use in cardiac surgery to maintain its flexibility. While in thoracic and abdominal wall reconstruction flexibility is not important.
Although the inlay technique is the simplest form of repair it has a disadvantage of lacking fixation of the implant by intra-abdominal pressure due to minimal surface area of contact between the implant and the adjacent tissue which lead to high relapse rates. Therefore, underlay technique is currently considered the best method due to the position of the implant behind the rectus muscles where the force of abdominal pressure holds the prosthesis against the deep surface of the abdominal muscle wall. Similar observations were reported by [20,21].
Various synthetic biomaterials such as carbon mesh, carbon fibre and nylon mesh have been used for the reconstruction of hernias in ruminants in the clinical cases [22,24]. Acellular dermal matrix has been successfully used for the reconstruction of hernias in goats [25]. Decellularised porcine and bubaline aorta have been used for construction of abdominal hernias in rats [26] and a cellular aortic matrix for reconstruction of abdominal hernias in calves [27] respectively, with excellent results. The results of the present study indicated that GBP and GTV serve as a good hernioplastic patch and viable option for the repair of abdominal hernias in farm animals.
References
- Lai JY, Chang PY, Lin JN (2003) Body wall repair using small intestinal submucosa seeded with cells. J Pediatr Surg 38: 1752-1755. [Crossref]
- Baxter GM (2004) Hernias/ Umbilicus. In farm animal Surgery. Fubini SL, Ducharme NG. Missouri; Saunders P. 477-484.
- Burger JW, Luijendijk RW, Hop WC, Halm JA, Verdaasdonk EG, et al. (2004) Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg 240: 578-583. [Crossref]
- Gangwar AK, Sharma AK, Kumar N, Kumar N, Maiti SK, et al. (2006) Acellular dermal graft for repair of abdominal wall defects in rabbits. J S Afr Vet Assoc 77: 79-85. [Crossref]
2021 Copyright OAT. All rights reserv
- Amid KP (1997) Classification of biomaterials and their related complications in abdominal wall hernia surgery. Hernia 1: 15-21.
- Marques A, Ademar L, Lucia M, Eliazabeth B, Marco T, et al. (1995) Retrospective study of the use of bovine pericardium, dura mater, and poly propylene mesh as reinforcement materials in abdominal and thoracic wall reconstruction. Current Therapeutic Research 56: 492-497.
- Abouelnasr KS, Zaghloul AE, Karrouf GI (2014) Comparative evaluation of glycerolized bovine pericardium implant with prolene mesh for repairing of abdominal wall defect in dogs. Iran Jl Vet Res 15: 211-217.
- Karrouf G, Zaghloul A, Abou-Alsaud M, Barbour E, Abouelnasr K (2016) Prosthetics and Techniques in Repair of Animal's Abdominal Wall. Scientifica (Cairo) 2016: 9463186. [Crossref]
- Hafeez YM, Zuki AB, Loqman MY, Noordin MM, Norimah Y (2005) Comparative evaluations of the processed bovine tunica vaginalis implant in a rat model. Anat Sci Int 80: 181-188. [Crossref]
- Fretz PB, Hamilton GF, Barber SM, Ferguson JG (1983) Management of umbilical hernias in cattle and horses. J Am Vet Med Assoc 183: 550-552. [Crossref]
- Kaneps AG (1992) Hernia, In: Auer.J.A (Ed): Equine surgery. W.B. Saunders. Co., Philadelphia 415-422.
- Mosbah E, Karrouf GIA (2006) Surgical management of certain umbilical affections in calves and foals. Minufiya Vet J 4: 249-270.
- Attinger CE, Bulan E, Blume PA (2000) Surgical débridement. The key to successful wound healing and reconstruction. Clin Podiatr Med Surg 17: 599-630. [Crossref]
- Al-Sobayil FA, Ahmed AF (2007) Surgical treatment for different forms of hernias in sheep and goats. J Vet Sci 8: 185-191. [Crossref]
- Krishnamurthy D (1996) Hernia. In Tyagi, RPS.; Singh, JIT., (Ed) Ruminant surgery. GBS Publishers and Distribution. India 225-237.
- Zaghloul AEI, Mosbah, E (2006) Reconstruction of abdominal wall defect by using bovine pericardium bioprosthesis. Mansoura Vet Med J 8: 111.
- Scott EA (1979) Repair of incisional hernias in the horse. J Am Vet Med Assoc 175: 1203-1207. [Crossref]
- Kader NH, Abass BT, Al-Sadi HI (2005) A comparative experimental study of the use of tunica vaginalis and pericardium as allograft for hernioplasty in sheep. Iraqi J Vet Sci 19: 57-70.
- James NL, Poole-Warren LA, Schindhelm K, Milthorpe BK, Mitchell RM, et al. (1991) Comparative evaluation of treated bovine pericardium as a xenograft for hernia repair. Biomaterials 12: 801-809. [Crossref]
- Reilingh VTS, Geldere VD, Langenhorst B, Jong D, Wilt GJ, et al. (2004) Repair of large midline incisional hernias with polypropylene mesh: comparison of three operative techniques. Hernia 8: 56-59. [Crossref]
- Ko R, Kazacos EA, Snyder S, Ernst DM, Lantz GC (2006) Tensile strength comparison of small intestinal submucosa body wall repair. J Surg Res 135: 9-17. [Crossref]
- Kumar N, Kinjavdekar P, Aithal HP, Amarpal AM, Pawde AM (2002) Surgical management of unusual large hernia with nylon mesh: A report of two cases. IntasPolivet 3: 86-87.
- Gangwar AK, Sharma AK, Kumar N, Devi KS (2008) Use of braided carbon fibres in the repair of umbilical hernia in bovines. Ind Vet J 85: 430-431
- Devi KS, Gangwar AK, Singh HN, Kumar N (2010) Studies on carbon mesh repair of congenital umbilical hernia in bovine. Ind Vet J 87: 139-141.
- Gangwar AK, Sharma AK, Kumar N, Maiti SK, Kumar N (2004) Xenogenicacellular dermal graft for the repair of ventral hernia in a non-descript goat. Ind Vet Med J 28: 95-96.
- Bellows CF, Jian W, McHale MK, Cardenas D, West JL, et al. (2008) Blood vessel matrix: a new alternative for abdominal wall reconstruction. Hernia 12: 351-358. [Crossref]
- Kumar V, Kumar N, Gangwar AK, Saxena AC (2013) Using acellular aortic matrix to repair umbilical hernias of calves. Aust Vet J 91: 251-253. [Crossref]
- EU Hernia Trialists Collaboration (2000) Mesh compared with non-mesh methods of open groin hernia repair: systematic review of randomized controlled trials. Br J Surg 87: 854-859. [Crossref]




