Abstract
Neuronal voltage-gated calcium channels (VGCCs) including Cav2.2, mediate the mechanisms involved in the presynaptic release of neurotransmitters. The role of Cav2.2 in neural circuits underlying depression remains poorly understood. In this study, intracerebroventricular injection of the Cav2.2 inhibitor ω-conotoxin GVIA (5 pg/side) in mice increased depression-like behavior in the forced swimming and tail suspension tests. These results suggest that Cav2.2-mediated signaling plays a role in depressive behaviors.
Key words
Cav2.2, depression, forced swimming test, ω-Conotoxin GVIA, tail suspension test
Introduction
Voltage-gated Ca2+ (Cav) channels play an important role in regulating diverse neuronal functions attributed to elevated intracellular Ca2+ concentrations [1,2]. Cav channels are molecular complexes comprising α1, β, α2-δ, and γ subunits [1]. The α1 subunit is essential for channel function and determines fundamental channel properties [1]. Genes encoding 10 pore-forming α1 subunits and several splice variants have been identified and characterized [3].
At the presynaptic terminal, Cav2.2 (N type) channels mediate Ca2+-dependent exocytotic release of neurotransmitters [4]. Ca2+ influx via these channels triggers neurotransmitter release in a cooperative process with other components of the vesicle fusion machinery [5]. Given the pivotal role of Ca2+ channels in controlling neurotransmitter release, defects in the expression, localization, structure, or modulation of presynaptic Ca2+ channels may result in aberrant synaptic signaling, leading to various patterns of neural network dysfunction. Cav2.2 channels have been reported to influence the release of dopamine [DA; 6–8], serotonin [5-HT; 9], glutamate [10], gamma-aminobutyric acid [11], acetylcholine [12], and norepinephrine [NE; 13] from central neurons. In terms of clinical relevance, imbalances of neurotransmitters are strongly associated with depression [14]. According to the monoamine hypothesis, depression can be ascribed to deficits in the major monoamine neurotransmitters (DA, 5-HT, and NE). As Cav2.2 channels are involved in the regulation of neurotransmitter release, administration of a Cav2.2 blocker is expected to result in depressive behavior.
In mice that received intracerebroventricular (i.c.v.) injections of ω-conotoxin GVIA, a Cav2.2 inhibitor, baseline levels of DA and 5-HT were reduced in the striatum and frontal cortex [15]. The Cav2.2 inhibitor also induced depressive behavior, as measured by the forced swimming test [16] and tail suspension test [17].
In the present study, the relationship between Cav2.2-mediated signaling and depression was investigated further. Mice were treated with i.c.v. injections of ω-conotoxin GVIA, and depression was assessed using forced swimming and tail suspension tests.
Materials and methods
Mice
All animal procedures were approved by the Animal Experiments Committee of Shanghai Jiao Tong University and RIKEN. C57BL/6J mice were provided by Charles River Japan (Kanagawa, Japan). The mice were given free access to water and food pellets (CRF-1; Oriental Yeast Co. Ltd., Tokyo, Japan) and housed under a 12/12-h light/dark cycle (lights on from 08:00 to 20:00) at 23 ± 1°C and 55 ± 5% humidity. We used separate groups of 2-month-old male mice for each of the behavioral tests. All experiments were conducted by investigators blinded to the treatment conditions at light phase.
Infusion
For the infusion studies, the Cav2.2 blocker, ω-conotoxin GVIA (100 pg/μL, Peptide Institute, Osaka, Japan) was dissolved in saline (vehicle). The drug dose was determined based on a previous report [15,18,19]. Non-treated mice received an equivalent volume of vehicle. Under anesthesia and using standard stereotaxic procedures, stainless-steel guide cannulae (22-gauge) were implanted into the lateral ventricle (posterior to bregma, -0.34 mm; lateral to midline, ±0.9 mm; ventral from the dura, −2.3 mm), and mice were allowed to recover for at least 1 week following surgery. The mice were briefly anesthetized with isoflurane to facilitate insertion of the injection cannula (26-gauge). Infusion into the lateral ventricle (0.1 μL/side) was accomplished at a rate of 0.05 μL/min 30 min before behavioral testing. The injection cannula was left in place for 2 min following infusion.
Open field test
Motor activity was measured by placing individual animals in a clear Plexiglas box (L × W × H: 30 × 20 × 15 cm). The box was then positioned in a frame on which infrared beams (Scanet SV-10, Tokyo, Japan) were mounted. The light intensity in the experimental room was 60 lux. Beam interruptions were summed in 20 min.
Tail-suspension test
The apparatus consisted of a non-transparent compartment (L × W × H: 15.0 × 16.0 × 25.0 cm) with a hook (4.0 cm in length). The distance between the hook and floor was 21 cm. Each mouse was hung from the hook using adhesive tape placed 2 cm from the end of its tail, and its behavior was recorded with a video camera for 7 min. The immobility time was evaluated between the 2nd and 7th min. The light intensity in the experimental room was 150 lux. The parameter recorded was the total amount of time (s) spent immobile.
Forced swimming test
Each mouse was placed in a 19-cm glass cylinder (11.0 cm in diameter) containing 13 cm of water at 23 ± 1°C. A mouse was deemed immobile when it floated and its hindlimbs appeared immobile, with only small movements of the forepaws used to keep the head above water. The light intensity in the experimental room was 150 lux. The behavior was recorded with a video camera for 7 min. Immobility was recorded between the 2nd and 7th min. The parameter recorded was the total amount of time (s) spent immobile.
Histology
Histological verification of the cannula locations was performed at the end of behavioral testing. Mice were perfused transcardially with 0.9% saline, followed by 4% paraformaldehyde (PFA). After extraction from the skull, the brains were post-fixed in 4% PFA and then transferred to a 30% sucrose solution until sectioning. Coronal sections (40 μm thick, taken every 120 μm) were cut on a cryostat (-16°C) and mounted on glass microscope slides. After drying, the sections were stained with cresyl violet.
Statistical analysis for behavioral results
The data are presented as the mean ± standard error of the mean (SEM). Statistical analyses were conducted using Excel Statistics 2006 (SSRI, Tokyo, Japan). The data were analyzed using analysis of variance (ANOVA). Tukey’s test was performed when appropriate. The results were considered significant at 5% or lower probability of error.
Results
This study examined the effect of ω-conotoxin GVIA on depressive behavior. Two groups of male mice were given i.c.v. injections of either ω-conotoxin GVIA (5 pg/side) or vehicle.
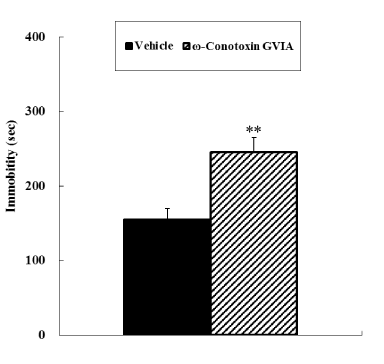
In the open field test, no significant difference was observed in motor activity between vehicle-injected and ω-conotoxin GVIA-injected mice (Figure 1). In both the tail suspension and forced swimming tests, ω-conotoxin GVIA-injected mice had significantly longer immobile times than vehicle-injected mice (Figure 2 and 3).
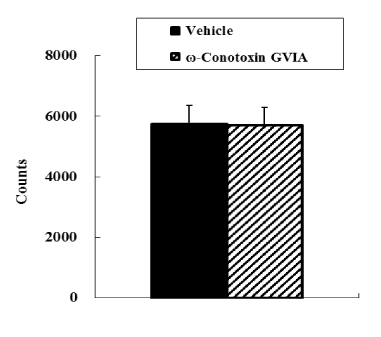
Figure 1. Open field test. The vehicle-injected or ω-Conotoxin GVIA-injected mice (n = 10 each) were allowed to explore the field freely for 20 min.
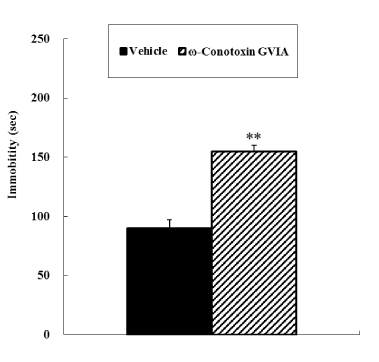
Figure 2. Tail suspension test. The vehicle-injected or ω-Conotoxin GVIA-injected mice (n = 10 each) were suspended by the tail. Time spent immobile (s) was evaluated during the 2nd to 7th minutes. The data are presented as means ± standard error of the mean (SEM). **P <0.01 compared with the appropriate control (Tukey’s test).
2021 Copyright OAT. All rights reserv
Figure 3. Forced swimming test. The vehicle-injected or ω-Conotoxin GVIA-injected mice (n = 10 each) were released in the apparatus and time spent immobile was evaluated during the 2nd to 7th minutes. The data are presented as means ± standard error of the mean (SEM). **P <0.01 compared with the appropriate control (Tukey’s test).
Mice with injection needle placements outside of the boundaries of the target areas were excluded from behavioral analyses (data not shown).
Discussion
The neurotransmission of monoamines is thought to control emotional behavior. Biological research in depression currently involves many aspects of neurotransmitter, hormone, and vitamin metabolism, with metabolism of monoamine neurotransmitters being a major area of interest for over 40 years [20–23]. Neuronal Cav channels, including Cav2.1 and Cav2.2, are predominantly expressed at presynaptic neuronal terminals throughout the central nervous systems [9]. Cav channels mediate the release of neurotransmitters that are involved in depression; however, the role of different Cav channels, specifically Cav2.2, in the neural circuits underlying depression has not been explored. In the present study, we investigated the relationship between Cav2.2-mediated signaling and depression in mice that received i.c.v. injections of the Cav2.2 blocker ω-conotoxin GVIA.
We first examined the effect of ω-conotoxin GVIA on motor activity by assessing immobility in the forced swimming and tail suspension tests. The open field test revealed no significant difference in motor activity between vehicle-injected and ω-conotoxin GVIA-injected mice. In a previous study, we examined the impact of a subtle disruption of Cav2.2 channel functioning on motor activity using the activity wheel test [24]. Cav2.2 channel knockout mice showed normal activity during the light phase and increased activity during the dark phase. In the present study, conducted in the light phase, Cav2.2 channel-dependent signaling had no effect on spontaneous activity in mice. In the forced swimming and tail suspension tests, ω-conotoxin GVIA-injected mice had significantly longer immobile times (i.e., increased depression-like behavior) compared with vehicle-injected mice. Overall, the results indicate that Cav2.2 channel-dependent signaling has an influence on depressive behaviors.
In our previous study, baseline levels of DA and 5-HT were reduced in the striatum and frontal cortex in mice given ω-conotoxin GVIA [15]. Although emotional behavior may be affected by multiple neurotransmitter systems, our results suggest that Cav2.2 channel dysfunction and subsequent decreases in DA and 5-HT may be at least partially responsible for the observed depressive behavior in ω-conotoxin GVIA-injected mice.
In conclusion, inhibition of Cav2.2-mediated signaling by the specific Cav2.2 blocker ω-conotoxin GVIA was found to induce behavioral deficits in the forced swimming and tail suspension tests. As Cav2.2 influences the release of DA and 5-HT [15], abnormalities in Cav2.2-mediated signal transduction may play a role in the pathophysiological mechanisms underlying depression. Additional electrophysiological studies of neurotransmitter release will help to elucidate the relationship between Cav2.2 signaling and depression.
Acknowledgments
This work was supported by China 973 project (2010CB529604) and National Scientific Foundation of China (81271511 and 30900432).
Conflicts of interest
The authors declare no competing interests.
Authors’ contributions
WL and ET designed and supervised the research, and wrote the manuscript. YZ and KN performed the surgeries and behavioral experiments. All authors read and approved the final version of the manuscript.
References
- Berridge MJ, Lipp P, Bootman MD (2000) The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1: 11–21. [Crossref]
- Liu L, Zwingman TA, Fletcher CF (2003) In vivo analysis of voltage-dependent calcium channels. J Bioenerg Biomembr 35: 671–865. [Crossref]
- Jarvis SE, Zamponi GW (2007) Trafficking and regulation of neuronal voltage-gated calcium channels. Curr Opin Cell Biol 19: 474–482. [Crossref]
- Yokoyama CT, Myers SJ, Fu J, Mockus S, Scheuer T, et al. (2005) Mechanism of SNARE protein binding and regulation of Cav2 channels by phosphorylation of the synaptic protein interaction site. Mol Cell Neurosci 28: 1–17. [Crossref]
- Catterall WA (1998) Structure and function of neuronal Ca2+ channels and their role in neurotransmitter release. Cell Calcium 24: 307–323. [Crossref]
- Herdon H, Nahorski SR (1989) Investigations of the roles of dihydropyridine and ω-conotoxin-sensitive calcium channels in mediating depolarization-evoked endogenous dopamine release from striatal slices. Naunyn Schmiedebergs Arch Pharmacol 340: 36–40.
- Turner TJ, Adams ME, Dunlap K (1993) Multiple Ca2+ channel types coexist to regulate synaptosomal neurotransmitter release. Proc Natl Acad Sci USA 90: 9518–9522. [Crossref]
- Woodward JJ, Rezazadeh SM, Leslie SW (1988) Differential sensitivity of synaptosomal calcium entry and endogenous dopamine release to ω-conotoxin. Brain Res 475: 141–145.
- Foehring RC (1996) Serotonin modulates N- and P-type calcium currents in neocortical pyramidal neurons via a membrane-delimited pathway. J Neurophysiol 75: 648–659. [Crossref]
- Luebke JI, Dunlap K, Turner TJ (1993) Multiple calcium channel types control glutamatergic synaptic transmission in the hippocampus. Neuron 11: 895–902. [Crossref]
- Horne AL, Kemp JA (1991) The effect of ω-conotoxin GVIA on synaptic transmission within the nucleus accumbens and hippocampus of the rat in vitro. Br J Pharmacol 103: 1733–1739.
- Wessler I, Dooley DJ, Werhand J, Schlemmer F (1990) Differential effects of calcium channel antagonists (ω-conotoxin GVIA, nifedipine, verapamil) on the electrically-evoked release of [3H] acetylcholine from the myenteric plexus, phrenic nerve and neocortex of rats. Naunyn Schmiedebergs Arch Pharmacol 341: 288–294.
- Dooley DJ, Lupp A, Hertting G, Osswald H (1998) ω-Conotoxin GVIA and pharmacological modulation of hippocampal noradrenaline release. Eur J Pharmacol 148: 261–267.
- Blier P (2013) Neurotransmitter targeting in the treatment of depression. J Clin Psychiatry 74: 19–24. [Crossref]
- Zhou Y, Niimi K, Li W, Takahashi E (2015) Inhibition of Cav2.2-mediated signaling induces sensorimotor gating deficits. Integr Mol Med 2: 256–260.
- Porsolt RD, Bertin A, Jalfre M (1978) "Behavioural despair" in rats and mice: strain differences and the effects of imipramine. Eur J Pharmacol 51: 291–294. [Crossref]
- Steru L, Chermat R, Thierry B, Simon P (1985) The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology 85: 367–370. [Crossref]
- Zhou Y, Niimi K, Li W, Takahashi E (2015) Disruption of spatial cognition by intra-accumbens injection of Cav2.2 inhibitor. Integr Mol Med 2: 109–111.
- Zhou Y, Niimi K, Li W, Takahashi E (2015) Spatial short-term memory regulated by Cav2.2-mediated NMDA receptor signaling. Integr Mol Med 2: 131–134. [Crossref]
- Rodgers RJ, Nikulina EM, Cole JC (1994) Dopamine D1 and D2 receptor ligands modulate the behaviour of mice in the elevated plus maze. Pharmacol. Biochem. Behav 49: 985–995. [Crossref]
- Zhou QY, Palmiter RD (1995) Dopamine-deficient mice are severely hypoactive, adiposic, and aphagic. Cell 83: 1197-1209. [Crossref]
- Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, et al. (1999) Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science 283: 397–401. [Crossref]
- Zhuang X, Gross C, Santarelli L, Compan V, Trillat AC, et al. (1999) Altered emotional states in knockout mice lacking 5-HT1A or 5-HT1B receptors. Neuropsychopharmacology 52S–60S (2 supplement). [Crossref]
- Nakagawasai O, Onogi H, Mitazaki S, Sato A, Watanabe K, et al. (2010) Behavioral and neurochemical characterization of mice deficient in the N-type Ca2+ channel alpha1B subunit. Behav Brain Res 208: 224–230. [Crossref]



 2021 Copyright OAT. All rights reserv
2021 Copyright OAT. All rights reserv