Abstract
Aim/Introduction
To detect salivary glucose levels and for the determination, if salivary glucose levels could be used as a non-invasive tool to monitor glycemic control in diabetics. Furthermore, the evaluation of relationship between salivary glucose levels and candidal carriage alongwith identification of strain diversity and antifungal susceptibility was also carried out.
Materials and methods
90 subjects with age range of 40-60 years were selected. Diabetic status was determined by estimation of random non-fasting plasma glucose levels and glycosylated hemoglobin levels. Un-stimulated and stimulated saliva was analyzed for glucose levels. An oral rinse sample with Phosphate buffered saline was used to assess candidal colonization. The candidal isolates were identified and tested for in-vitro antifungal susceptibility using disc diffusion method.
Results
Salivary glucose levels were significantly higher in diabetics and there was significant correlation between salivary glucose and random non-fasting plasma glucose. Candidal carriage was higher in diabetics with C. Albicans being the most frequently identified yeast in all groups, but subjects carried other fungal species as well. The antifungal susceptibility revealed that the Candida had different rates of resistance to the tested antifungal drugs except Amphotericin B.
Conclusions
Salivary glucose levels could possibly be used as a non-invasive tool to monitor glycemic control in diabetics. The candidal carriage is increased in the diabetics. The most effective antifungal drug was Amphotericin B. Culture and sensitivity testing would add to the value of selecting appropriate antifungal drug as to prevent emergence of drug resistance.
Key words
salivary glucose, candidal carriage, antifungal susceptibilty
Introduction
Diabetes Mellitus is a clinical syndrome characterised by hyperglycemia due to absolute or relative deficiency of insulin. It occurs worldwide and the incidence is rising [1]. World Health Organisation statistics show that India has the highest population of Diabetics amongst all the countries in the world. This is estimated to increase to 79.4 million by the year 2030 [2].
Hyperglycaemia which is considered as the hall mark of Diabetes Mellitus, has also been consistently documented to be associated with altered salivary composition and function [3]. Basement membrane permeability of the parotid gland is reported to be higher in Diabetes, and this results in raised percolation of components such as glucose, amylase, proteins, etc, from blood, thus raising their levels in saliva. The resulting altered salivary composition disrupts the homeostasis of the oral cavity, making it susceptible to various oral ailments [4].
Oral candidasis is a common opportunistic fungal infection of the oral cavity caused by an overgrowth of candidal species, the most common being Candida Albicans [5]. Diabetes Mellitus can impair the function of polymorphonuclear leukocytes which may predispose diabetic patients to a greater risk of oral candidal infections [6]. These fungal species are common components of the human oral flora [5]. Other Candidal species such as C. Glabrata, C. Parapsilosis, C. Krusei, and C. Tropicalis can also be isolated from the saliva of diabetic patients [7].
Candidal infections are treated by antifungal agents. The most common antifungal drugs in current clinical use, for treatment of oral candidasis are polyenes such as Amphotericin B and Nystatin and azoles such as Fluconazole, Ketoconazole, and Itraconazole; mainly used topically. However, the therapeutic and prophylactic use of antifungal agents has given rise to an alarming number of cases of resistance on those drugs [8].
This makes accurate identification of strains isolated from diabetic patients especially important, because they are more likely to carry species other than C. Albicans, which might not be sensitive to certain antifungal agents. In addition, culture and sensitivity testing will go a long way in the selection of appropriate antifungal drugs, rather than prescribing any type of antifungal drug just on the basis of any clinical manifestation of candidal infection [8].
Materials and method
90 subjects of either sex reporting to the Department of Oral Medicine and Radiology of Maharishi Markandeshwar College of Dental Sciences & Research, Mullana, India were selected. All subjects were informed and explained about the study and a signed informed consent was taken. The study was conducted as per Helsinki declaration (1964) & ethical clearance was taken from the ethical committee of the University. The data such as age, sex, any significant medical history, was recorded in a specially formatted proforma and clinical examination was then carried out.
All subjects were in the age range of 40-60 years and were divided into 3 groups, with 30 subjects in each group. Group I (Controlled Diabetic subjects) comprised of subjects who were being treated for diabetes and had random non-fasting plasma glucose (RNFPG) values >120 mg/dL and <200 mg/dL and fasting HbA1C level below 7.0 mmol/L [9]. Group II (Uncontrolled Diabetic subjects) comprised of subjects who were being treated for diabetes and had random non-fasting plasma glucose (RNFPG) values >200 mg/dL and fasting HbA1C level above 7.0 mmol/L [9]. Group III (Nondiabetic subjects) comprised of subjects who had random non-fasting plasma glucose (RNFPG) values within 80-120 mg/dL.
Subjects with any other systemic disorders except for hypertension were excluded from the study. So were the subjects, who chewed tobacco or had other chewing habits, which received antibiotics or steroid therapy or had been using antiseptic mouthwash during the last three weeks and those who were unwilling to participate in the study.
The subjects of all three above mentioned groups were subjected to plasma glucose and salivary glucose levels tests. The tests were again performed once after three days and then again 1 week later.
Sample collection and assesment of plasma and salivary glucose levels
Blood- 2 mL peripheral venous blood was collected from every subject.
Saliva- The subjects were asked to have their breakfast & to abstain from eating for 2 hours before sample collection. All salivary samples were collected 2 hours after the subject’s breakfast. Unstimulated saliva was collected using a spit technique. The subject was asked to sit in the dental chair with head tilted forward & instructed not to speak, swallow, or do any head movements during the procedure, or swallow any saliva if present in the mouth. The subject was instructed to spit in a sterile graduated container every minute, for 10 minutes. Stimulated saliva was collected using 2% food-grade citric acid applied to the dorsolateral surface and tip of tongue every 30 seconds, and the subject was asked to spit the saliva as it pooled in the mouth, into sterile container without swallowing, for 3 minutes [5].
Glucose levels of plasma, stimulated & un-stimulated saliva were measured by glucose-oxidase method in semi-automated analyzer. Glycosylated HbA1c levels were measured using the ion-exchange resin method.
Saliva sampling for oral candidal carriage, strain diversity and antifungal susceptibility of candida
Saliva sampling for oral candidal carriage, strain diversity and antifungal susceptibility of candida was performed using the oral rinse technique. The subjects were asked to rinse their mouth for 60 seconds with 10 mL sterile phosphate-buffered saline. The rinse was immediately concentrated by centrifuging at 1700 rpm for 10 minutes. Supernatant was discarded & inoculating loop was used to spread the sample onto Sabouraud dextrose agar with chloramphenicol (10 mg/mL). It was incubated at 37˚C and growth was observed after 48 hours [5,10]. The presence of creamy white, pasty, opaque and smooth colonies suggested Candida [11]. Candidal growth on the agar was confirmed by Gram staining. Candida is a gram positive budding yeast cell and stains dark blue/purple with the Gram’s staining [11].
For strain diversity, a recently developed system, the CHROMagar, which uses chromogenic substances, which results in formation of differently colored colonies was used [11,12]. Inoculating loop was used to spread the sample onto CHROMagar plates. The plates were incubated at 37˚C for 48 hours and growth was observed after that [10,13,14]. The presumptive identification of Candida species was based on the criteria proposed by Odds and Bernaerts (1994), who described the species by the color of the colony [12,15], as follows:
C. Albicans colonies → green color
C. Tropicalis colonies → dark-blue to blue-gray color, surrounded by a dark/ pink halo
C. Glabrata colonies → white/dark pink/purple range of colors
C. Krusei colonies → pale pink color and downy/rough appearance with pale edges [12,14,15].
Antifungal Susceptibility Testing was done using the National Committee for Clinical Laboratory Standards, 2004 method for antifungal disc diffusion susceptibility for yeasts with approved guideline M44-A [16]. The following antifungal discs were used on sterile Mueller-Hinton + 2% Glucose and 0.5 μg/mL Methylene Blue Dye medium: Fluconazole 25 µg, Ketoconazole 50 µg, Itraconazole 30 µg, Miconazole 30 µg, Amphotericin 50 µg.
The zone diameter was measured to the nearest whole millimeter at the point at which there was a prominent reduction in growth [17]. Zone diameter interpretive criteria were used to categorize accurately the levels of susceptibility of organisms to various antifungals using the chart given by the manufacturer (HiMedia).
Results
Data entry, database management and all statistical analysis were performed with Statistical Package for the Social Sciences (SPSS) software. The study population which consisted of 90 subjects were divided into three groups. A highly significant correlation was observed in the mean HbA1c levels between the Controlled Diabetic and the Uncontrolled Diabetic groups, with a p value of <0.001.
The mean Random Non Fasting Plasma Glucose (RNFPG), unstimulated (USSG) and stimulated salivary glucose (SSG) levels were higher in the diabetic patients than in the control group. Among the diabetic groups, these were higher in the uncontrolled diabetic group (Graph 1).
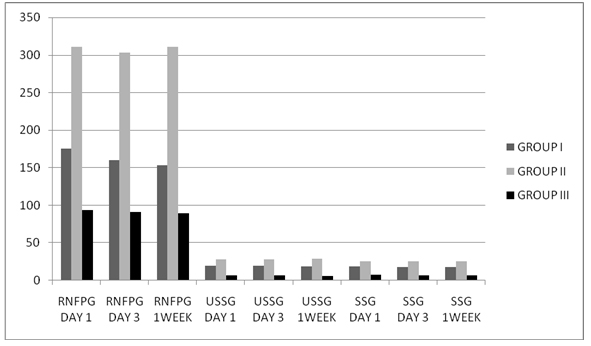
Graph 1. Mean RNFPG, USSG & SSG levels in study population.
The mean RNFPG level on Day 1 in Group I was 175.15 mg/dl, in Group II it was 311.12 mg/dl and in Group III it was 94.07 mg/dl. On Day 3, the mean RNFPG level in Group I was 159.75 mg/dl, in Group II it was 303.34 mg/dl and in Group III it was 90.74 mg/dl. At the end of one week, the mean RNFPG level in Group I was 152.90 mg/dl, in group II it was 310.62 mg/dl and in Group III it was 89.30 mg/dl.
The mean USSG on Day 1 in Group I was 19.49 mg/dl, in Group II it was 28.20 mg/dl and in Group III it was 7.01 mg/dl. On Day 3, the mean USSG level in Group I was 19.31 mg/dl, in Group II it was 27.73 mg/dl and in Group III it 6.72 mg/dl. At the end of 1 week, the mean USSG level in Group I was 18.81 mg/dl, in Group II it was 28.59 mg/dl and in Group III it was 6.38 mg/dl.
The mean SSG level on Day 1, in Group I was 18.39 mg/dl, in Group II it was 25.62 mg/dl and in Group III it was 7.73 mg/dl. On Day 3, the mean SSG level in Group I was 17.93 mg/dl, in Group II it was 25.87 mg/dl and in Group III it was 7.18 mg/dl. At the end of 1 week, the mean SSG level in Group I was 17.60 mg/dl, in Group II it was 25.85 mg/dl and in Group III it was 7.10 mg/dl.
A highly significant correlation was observed in the mean RNFPG, USSG and SSG levels on Day 1, Day 3 and 1 week, between all the groups, with p value of <0.001. Both the USSG and the SSG showed significant positive correlation with RNFPG levels in the study population, in the diabetic group. In Group I, the USSG & SSG levels were significantly correlated with the RNFPG level with p value of 0.024 & 0.028 respectively. In Group II, the USSG and SSG level had a highly significant correlation with RNFPG level with p value of 0.000. In Group III, the USSG level had no significant correlation with the RNFPG level with p value of 0.072. But SSG level showed a significant correlation with the RNFPG level with p value of 0.049. The differences between USSG and SSG on Day 1, Day 3 and 1 week, within the groups were also significant.
Candidal carriage among the groups showed that there was increased carriage amongst the diabetic group as compared to the control group. However, in spite of the increased candidal carriage in the diabetic group comprising of 60 subjects (33.3%), the frequency of detecting positive candidal growth was not significant with p value of 0.96 as compared to the control group which comprised of 30 subjects (16.7%) (Graph 2). Within the diabetic groups, candidal carriage was positive in 9 subjects (30%) of Group I as compared to 11 subjects (36.7%) in Group II. But the frequency of detecting positive candidal growth in Group II was not significant as compared to Group I with p value of 0.584.
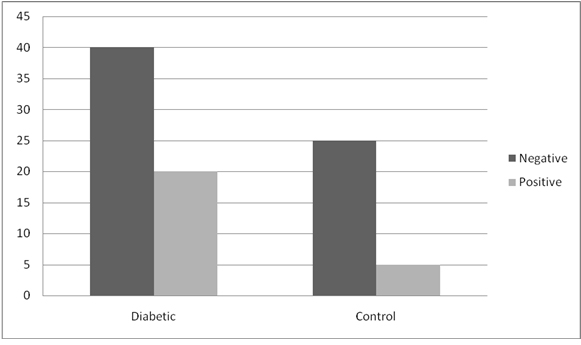
Graph 2. Candidal carriage between groups.
When a correlation between candidal carriage and salivary glucose levels was made, it was found that the candidal carriage showed a highly significant correlation with salivary glucose only in Group I with p value of 0.006 for mean USSG level and p value of 0.008 for mean SSG level. In Group II and III, there was no significant correlation between candidal carriage and mean USSG level with p value of 0.747 and 0.978, respectively or the mean SSG level with p value of 0.606 and 0.889, respectively.
While analyzing strain diversity of Candida in the study population, it was seen that in all the groups, the most frequently isolated yeast was Candida albicans. The prevelance was 77.8% in Group I, 90.9% in Group II and 80% in Group III. The remaining isolated yeasts were found more frequently in the diabetic patients as compared to the control group. C. Krusei was found in 22.2% of the subjects in Group I and 20% of the subjects in Group III. C. Tropicalis was found in exclusively in Group II in 9.1% of the subjects (Graph 3).
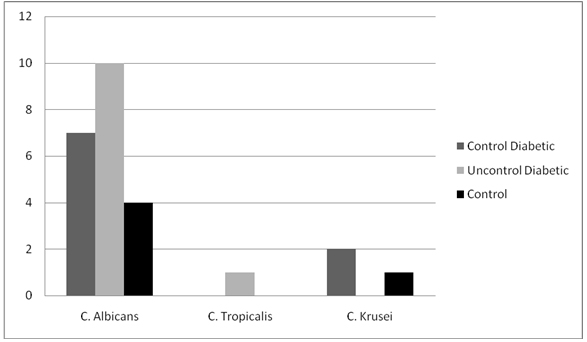
Graph 3. Strain diversity of Candida.
The in-vitro antifungal susceptibility of Candida revealed that the Candida isolated from the subjects had different rates of resistance to the five tested antifungal drugs, except Amphotericin B, against which they had no resistance. However, when subjects in different groups were compared, there was no statistical significant difference in the antifungal susceptibility (Table 1).
Antifungal agent | | | Controlled Diabetes | Uncontrolled Diabetes | Control | Total | P value |
Fluconazole 25 µg | Sensitive | Count | 3 | 3 | 1 | 7 | 0.938 |
% | 33.3% | 27.3% | 25.0% | 29.2% |
Resistant | Count | 6 | 8 | 3 | 17 |
% | 66.7% | 72.7% | 75.0% | 70.8% |
Ketoconazole 50 µg | Sensitive | Count | 3 | 1 | 1 | 5 | 0.403 |
% | 33.3% | 9.1% | 20.0% | 20.0% |
Resistant | Count | 6 | 10 | 4 | 20 |
% | 66.7% | 90.9% | 80.0% | 80.0% |
Itraconazole 30 µg | Sensitive | Count | 3 | 7 | 4 | 14 | 0.191 |
% | 33.3% | 63.6% | 80.0% | 56% |
Resistant | Count | 6 | 4 | 1 | 11 |
% | 66.7% | 36.4% | 20.0% | 44% |
Miconazole 30 µg | Sensitive | Count | 3 | 4 | 2 | 9 | 0.969 |
% | 33.3% | 36.4% | 40.0% | 36.0% |
Resistant | Count | 6 | 7 | 3 | 16 |
% | 66.7% | 63.6% | 60.0% | 64.0% |
Amphotericin B 50 µg | Sensitive | Count | 9 | 11 | 5 | 25 | NA |
% | 100% | 100% | 100% | 100% |
Resistant | Count | 0 | 0 | 0 | 0 |
% | 0% | 0% | 0% | 0% |
Table 1. In vitro antifungal susceptibility of Candida.
Discussion
Diabetes mellitus is assured to be one of the elementary challenges to health professionals for the twenty first century. The two key aspects of diabetic management are normalization of blood glucose level and its judicious monitoring; both of these need the patient’s regular compliance. The present method of blood glucose estimation needs the venepuncture, which is highly traumatic to the patients at times. Apart from physical trauma, the process also renders mental trauma and anxiety about the procedure to discourage the patients [18]. Hence, a non-invasive, simple and painless procedure, such as salivary glucose estimation could be preferable.
The use of saliva rather than blood for diagnosis has recently been promoted [19]. Two of the advantages of salivary assessment are its non-invasive collection and cost effectiveness for screening large populations [20].
The important criterion to choose the glucose in saliva to measure the blood glucose is that, saliva is said to be the ultra-filtrate of blood [18]. Glucose is one of the blood components that are transferable across the salivary gland epithelium in proportion to its concentration in blood [18].
In the present study, it was observed that the mean Random Non Fasting Plasma Glucose, un-stimulated and stimulated salivary glucose levels were higher in the diabetic patients than in the control group. Among the diabetic groups, these were higher in the uncontrolled diabetic group. These finding were similar to the finding of Shehla Amer, et al. [21] where they found out that the salivary samples of the non-diabetic control subjects did not show the presence of glucose even in the slightest concentrations, while the samples obtained from the diabetics showed significant concentrations of glucose in the saliva.
Our results were also comparable to the studies conducted by María Elena López, et al. [22] and Suleyman Aydin [19] where they reported that glucose levels were higher in diabetic subjects than in the control group. Arati S. Panchbhai [4] and S. Sathya priya, et al. [23] also reported analogous results from their respective studies.
However, there is divergence with respect to absolute values determined for salivary glucose concentration. It is contemplated that such differences can be due to differences in methods utilized to determine glucose and in saliva collection.
In our study, both the un-stimulated and stimulated salivary glucose levels showed significant positive correlation with random non-fasting plasma glucose levels in the study population, in the diabetic group. However, in the control group, the un-stimulated salivary glucose level had no significant correlation with the random non-fasting plasma glucose level but the stimulated salivary glucose level had a significant correlation. Hence, salivary glucose appears to be an indicator of serum glucose concentration in diabetic patients.
The results were similar to the findings of Radhika Sashikumar, et al. [5], Arati S. Panchbhai, et al. [18] and Panda Abikshyeet, et al. [24] who reported that there was significant positive correlation between salivary and plasma glucose levels.
In contrast to our study, a few other trials could not establish a correlation between salivary and serum glucose. In a study conducted by Carmen Carda, et al. [25] salivary glucose was only augmented in patients with poor metabolic control but the increase of the blood glucose levels was not related with an increase in the salivary glucose concentration. This might be due to the diversity in selection criteria of the samples and the difference in the design of study. Also, in a study conducted by P. Bakianian Vaziri, et al. [20] there was no significant difference in salivary IgA and glucose concentrations between type 1 and type 2 diabetic patients and their matched control subjects. These differences may be due to the diabetic status and glycemic control.
Candida species colonize mucosal surfaces of human beings during or soon after birth and risk of endogenous infection is ever present. As members of normal microbial flora, candida and related yeasts are endogenous opportunistic organisms and diabetics are vulnerable to develop opportunistic infections because of high glucose levels in tissues. The carriage rate of candida in the oral cavity has been different in various studies. This could be due to different methods of sampling [26]. Several previous studies have shown the prevalence of Candidal species is greater among diabetics than in normal persons [27].
The oral rinse sampling method was used as it was the most appropriate and sensitive technique for evaluating the overall yeast carriage as compared to imprint culture, swab or saliva sampling [7]. In accordance to previous studies, in the present study, it was observed that there was increased candidal carriage in the diabetic group, more in uncontrolled diabetics, as compared to the control group. However, the results were not statistically significant. Even though candidal carriage was higher in the uncontrolled diabetic group, there was no significant correlation with the salivary glucose. However, a highly significant correlation of candidal carriage with salivary glucose was seen in the controlled diabetic group.
The results were also in agreement to the studies conducted by BV Kumar et al. [28] and Faris Abdul Kareem Khazal et al. [27] who reported that carriage and colonization of Candida was more in diabetic patients when compared to non-diabetic subjects. Such carriage of Candida in the oral cavity of diabetes mellitus was independent of the type of anti-diabetic therapy the subjects were receiving or the glycemic control that was achieved. Even though the carriage rate of Candida was high, the results were not statistically significant.
Studies done on candidal colonization are often contradictory, which maybe the result of the variety of sampling techniques employed [8]. In a study conducted by James Guggenheimer, et al. [29] and Safia A. Al-Attas, et al. [8], it was reported that carriage rate or the frequency of detecting positive candidal growth was significantly higher in the diabetic patients than in the control group and the results were highly significant.
Microbiological testing in the field of Oral Medicine facilitates diagnosis, choice of therapy, treatment assessment, and risk evaluation [30]. In the present study, the most frequently isolated yeast was Candida albicans. The remaining isolates were found more frequently in the diabetic patients as compared to the control group. This was similar to the findings of Faris Abdul Kareem Khazal et al. [27], Barbara Dorocka-Bobkowska, et al. [31] and Safia A. Al-Attas, et al. [8] where C. Albicans was the most frequently isolated candidal species among the study groups, but the diabetic patients also carried other yeasts and candidal species which were C. Glabrata, C. Parapsilosis, C. Krusei and C. Tropicalis.
Based on the above findings we can say that the accurate identification of strains isolated from the diabetic patients while prescribing antifungal drugs is very important because they can carry species other than C. Albicans. Culture and sensitivity testing will add to the value of selecting the appropriate antifungal drug. In the present study, Candida isolated from the subjects had different rates of resistance to the five tested antifungal drugs, except Amphotericin B, against which they had no resistance. The highest resistance was for Ketoconazole followed by Fluconazole, Miconazole and Itraconazole. These findings were similar to the findings of the study conducted by Ragini Ananth Kashid, et al. [16] who reported that the antifungal susceptibility pattern showed that most Candida isolates had no resistance was seen for Amphotericin B. Similar findings were observed by Safia A. Al-Attas, et al. [8] who reported that oral yeasts isolated from diabetic patients displayed different rates of resistance from azole antifungal agents, mainly Miconazole and Fluconazole but no resistance against Amphotericin B and Nystatin.
Such findings confirmed the results of other researchers reporting the emergence of triazole resistance among different groups of immunocompromised patients in whom these agents were frequently used [8]. There is a need for periodic surveillance of antifungal susceptibility pattern of the prevalent Candida species, as it would enlighten the judicious use of antifungal drugs in such patients [16].
From this study, it can be concluded that the salivary glucose levels could be used as a non-invasive tool to monitor glycemic control in diabetics. In addition, the candidal carriage is more in the diabetic patients with C. Albicans being the most predominant species. Other species of Candida were found more frequently in the diabetic subjects as compared to the control subjects. If any antifungal is required to be given to a diabetic patient, it is recommended that the species of candida be identified and antifungal susceptibility be done. This will prevent incidence of resistance for various antifungal drugs, thereby, not rendering it useless for future generations. However, if antifungal susceptibility testing is not possible due to any reason, the drug which can be prescribed is Amphotericin B. The usefulness of various antifungal drugs can be conserved by this practice.
Acknowledgements
No conflict of interest has been observed among the authors.
References
- Walker BR, Ralston SH (2014) Davidson’s Principles & Practice of Medicine, Elsevier Churchill Livingstone, Diabetes Mellitus. (20th edn.) Elsevier, Canada.
- Wild S, Roglic G, Green A, Sicree R, King H (2004) Prevalence of Diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 27:1047–1053. [Crossref]
- Vernillo AT (2003) Dental considerations for the treatment of patients with diabetes mellitus. J Am Dent Assoc 134: 24S-33S. [Crossref]
- Panchbhai AS, Degwekar SS, Bhowte RR (2010) Estimation of salivary glucose, salivary amylase, salivary total protein and salivary flow rate in diabetics in India. J Oral Sci 52: 359-368. [Crossref]
- Sashikumar R, Kannan R (2010) Salivary glucose levels and oral candidal carriage in type II diabetics. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 109: 706-711. [Crossref]
- Javed F, Klingspor L, Sundin U, Altamash M, Klinge B, et al. (2009) Periodontal conditions, oral Candida albicans and salivary proteins in type 2 diabetic subjects with emphasis on gender. BMC Oral Health 12: 9:12. [Crossref]
- Samaranayake LP, MacFarlane TW, Lamey PJ, Ferguson MM (1986) A comparison of oral rinse and imprint sampling techniques for detection of yeast coliform and Staphlococcus aureus carriage in the oral cavity. J Oral Pathol 15: 386-388. [Crossref]
- Al-Attas SA, Amro SO (2010) Candidal colonization, strain diversity, and antifungal susceptibility among adult diabetic patients. Ann Saudi Med 30: 101-108. [Crossref]
- Bremenkamp RM1, Caris AR, Jorge AO, Back-Brito GN, Mota AJ, et al. (2011) Prevalence and antifungal resistance profile of Candida spp. oral isolates from patients with type 1 and 2 diabetes mellitus. Arch Oral Biol 56: 549-555. [Crossref]
- Beighton D1, Ludford R, Clark DT, Brailsford SR, Pankhurst CL, et al. (1995) Use of CHROMagar Candida Medium for Isolation of Yeasts from Dental Samples. J Clin Microbiol 33: 3025–3027. [Crossref]
- Mackie TJ (1989) Mackie & McCartney Practical Medical Microbiology. (14th edn.) Churchill Livingstone, Fungi.
- Odds FC, Bernaerts R (1994) CHROMagar Candida, a new Differential Isolation Medium for Presumptive Identification of Clinically Important Candida Species. J Clin Microbiol 32: 1923-1929. [Crossref]
- Rabelo GD, Noborikawa E, Siqueira CS, Silveira FRX, Lotufo MA (2010) Detection of single and mixed colonization of Candida species in patients with denture stomatitis. Braz J Oral Sci 3: 184-188.
- Vijaya D, Harsha TR, Nagaratnamma T (2011) Candida Speciation Using Chrom Agar. J Clin Diagnos Res 5: 755-757.
- Collier L, Balows A, Sussman M (1997) Topley & Wilson’s Microbiology and Microbial Infections. (9th edn.) Hodder Arnold, UK.
- Kashid RA, Belawadi S, Devi G, Indumati (2011) Characterisation and antifungal susceptibilty testing for candida species in a tertiary care hospital. J Health Sci Res 2: 1-7.
- National Committee for Clinical Laboratory Standards 2004. Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts; Approved Guideline. NCCLS document M44-A 24.
- Panchbhai AS (2012) Correlation of Salivary Glucose Level with Blood Glucose Level in Diabetes Mellitus. J Oral Maxillofac Res 3: e3. [Crossref]
- Aydin S (2007) A Comparison of Ghrelin, Glucose, Alpha-amylase and Protein Levels in Saliva from Diabetics. J Biochem Mol Biol 40: 29-35. [Crossref]
- Vaziri PB, Vahedi M, Mortazavi H, Abdollahzadeh S, Hajilooi M (2010) Evaluation of Salivary Glucose, IgA and Flow Rate in Diabetic Patients: A Case-Control Study. J Dent (Tehran) 7: 13-18. [Crossref]
- Amer S, Yousuf M, Siddiqui PQR, Alam J (2001) Salivary glucose concentrations in patients with diabetes mellitus – a minimally invasive technique for monitoring blood Glucose levels. Pak J Pharm Sci 14: 33-37. [Crossref]
- López ME Colloca ME, Páez RG, Schallmach JN, Koss MA (2003) Salivary Characteristics of Diabetic Children. Braz Dent J 14: 26-31. [Crossref]
- Priya SS, Bharani GO, Nagalingam M, Jayanthi M, Kanagavalli U (2011) Potential of Salivary Protein as a Biomarker in prognosis of Diabetes mellitus. J Pharmacy Res 4: 2228-2229.
- Abikshyeet P, Ramesh V, Oza N (2012) Glucose estimation in the salivary secretion of diabetes mellitus patients. Diabetes Metab Syndr Obes 5: 149-154. [Crossref]
- Carda C, Mosquera-Lloreda N, Salom L, Gomez de Ferraris ME, Peydró A (2006) Structural and functional salivary disorders in type 2 diabetic patients. Med Oral Patol Oral Cir Bucal 11: E309-E314. [Crossref]
- Bharathi M, Rani AU, Sandhya C (2012) A comparative study of carrier state of candida and its speciation in oral flora – among healthy individuals, persons with DM and HIV sero positive individuals. Our Dermatol Online 3: 102-106. [Crossref]
- Khazal FAK, Mahran A, Al-Hasnawi HH (2006) Oral Carriage Rate of Candida Species in Diabetic Patients. Al-Kindy Col Med J 3: 9-12.
- Kumar BV1, Padshetty NS, Bai KY, Rao MS (2005) Prevalence of Candida in the Oral Cavity of Diabetic Subjects. JAPI 53: 599-602. [Crossref]
- Guggenheimer J, Moore PA, Rossie K, Myers D, Mongelluzzo MB, et al. (2000) Insulin-dependent diabetes mellitus and oral soft tissue pathologies. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 89: 570-576. [Crossref]
- Martins M, Henriques M, Ribeiro AP, Fernandes R, Vania Goncalves, et al. (2010) Oral Candida carriage of patients attending a dental clinic in Braga, Portugal. Rev Iberoam Micol 27:119-124. [Crossref]
- Barbara Dorocka-Bobkowska, Anna Szumata-Kakol, Wiestaw Hêdzelek (2007) Characteristics of chosen features of yeast-like fungi in denture stomatitis in type 2 diabetes. Mikologia Lekarska 14: 41-45.



