Abstract
A 10 Hz rhythm is present in the occipital cortex when the eyes are closed (alpha waves), in the precentral cortex at rest (mu rhythm), in the superior and middle temporal lobe (tau rhythm), in the inferior olive (projection to cerebellar cortex), and in physiological tremor (underlying all voluntary movement). These are all considered resting rhythms in the waking brain which are “replaced” by higher frequency activity with sensorimotor stimulation. That is, the 10 Hz frequency fulcrum is replaced on the one hand by lower frequencies during sleep, or on the other hand by higher frequencies during volition and cognition. The 10 Hz frequency fulcrum is proposed as the natural frequency of the brain during quiet waking, but is replaced by higher frequencies capable of permitting more complex functions, or by lower frequencies during sleep and inactivity. At the center of the transition shifts to and from the resting rhythm is the reticular activating system, a phylogenetically preserved area of the brain essential for preconscious awareness.
Key words
alpha rhythm, mu rhythm, physiological tremor, readiness potential, reticular activating system, tau rhythm
Introduction
There is an indisputable difference between sensorial exposure and brain function in the real world and that which is studied in the laboratory. In the real world, the brain is under continuous sensory load and, therefore, always processing complex information. An animal exploring the natural environment is constantly bombarded with a myriad of sensations, exposed to multiple perceived threats, and forced to calculate fight-vs-flight responses, all while simultaneously planning the next meal. In contrast, a laboratory subject performing aspects of a banal protocol in the confines of a neat and sterile lab is exposed to “control” conditions wherein a unitary stimulus is presented at a defined point in time, a set-up contrived to elicit a fixed response. Juxtaposed the latter background is a subject whose main problem is that of falling asleep and a team of sensory and motor physiologists whose primary threat is succumbing surreptitiously to tunnel vision.
Admittedly, experimental tunnel vision is not problematic per se. Early sensorimotor investigators were tasked with understanding phenomena that seemed inconsistent and elusive, a situation that established a warrant for the “controlled” conditions requisite for the dissection of basic scientific principles. That is, the need to determine cause and effect necessitated experimental manipulations of the least common denominator, successful endeavors that elucidated the basic principles of brain function, particularly in regards to how the brain perceives simple stimuli and events, i.e., the “content of sensory experience.” Paradoxically, these reductionist approaches now seem anachronistically displaced amidst convergent evidence that the processing of sensory “content” is dramatically affected by background frequency states in the brain, the “context” under which we perceive and act.
For example, it is known that an identical auditory stimulus evokes a similar cortical response during waking and sleep. However, the perception of the stimulus varies as a function of the arousal state: A stimulus that can be perceived during waking cannot be perceived during sleep. Underlying this variation in response is a difference in the background frequency, or “context” of the brain, characteristic of waking and sleep states. During alert waking, it is possible to generate high frequency background activity (~40 Hz gamma band) to facilitate the recognition and integration of stimuli [1]. Yet while asleep, a low frequency background activity (~4 Hz delta band) predominates and, ultimately, negates the ability of the brain to recognize or associate meaning with the stimulus. Centered amidst this dichotomy are ~10 Hz alpha waves, a veritable fulcrum situated between functional states, a waveform that demarcates the functional shifts between large and small scale ensemble activity [2].
Knowledge of the aforementioned underscores the imperative for contemporary neuroscientists to consider how frequency context impinges upon the processing and output of sensorimotor phenomena. By corollary, consideration of the anatomy and physiology of structures that gives rise to the dynamic context of the brain becomes warranted. Accordingly, we review below in brief the occurrence of ~10 Hz rhythms in key brain regions and examine their putative role as a carrier frequency requisite for the facilitation of sensorimotor events. We offer evidence that the pedunculopontine nucleus (PPN), part of the reticular activating system (RAS), is a central structure involved in the functional pivot from low to high frequencies and then describe anatomical and physiological correlates of the PPN that permit this activity. Finally, we explicate the effects of alpha and gamma frequency aberrations in both health and disease.
Alpha frequencies and the RAS
The RAS plays a vital role in the modulation of frequency states that are crucial for arousal and, thereby, sensorimotor function during active waking. Of the RAS nuclei, the PPN plays a particularly prominent role in coordinating changes in firing patterns (or functional shifts) in key brain regions during sensorimotor events, as evident by alpha to beta/gamma transitions that are observed on electroencephalogram (EEG). Bolstering this notion is evidence that patients who perform self-paced wrist and ankle movements exhibit oscillatory shifts between alpha and beta band activity in the PPN [3]. Specifically, passive movements tend to increase alpha activity in the PPN whereas imagined movements tend to decrease it, suggesting that motor activity uncouples alpha phase locking [4]. Similar oscillations have been reported in the PPN in humans during gait [5].
Parallel work in animal models has shown that PPN neurons fire at ~10 Hz during cortical slow oscillations but also support nested gamma oscillations [6]. Our in vitro studies have shown that PPN cells exhibit a “resting” firing frequency in the 8-12 Hz range in the absence of stimuli, but fire maximally at gamma band frequency with the application of appropriately ramped depolarizing electrical stimuli [7-9]. Notably, these frequency patterns are altered markedly following manipulation of transmitter agonists [7] and temperature levels. Figure 1 shows the effects of temperature on PPN activity in the absence of pharmacological stimulation. Achievement of the 36°C threshold is essential for eliciting beta range spontaneous activity in the PPN. That is, higher frequencies manifest more fully at temperatures commensurate with basal body temperature, not in the frigid environment (<35°C) frequently used for in vitro recordings. This knowledge has prompted us to perform all of our in vitro recording studies at 36-37°C [8-14].
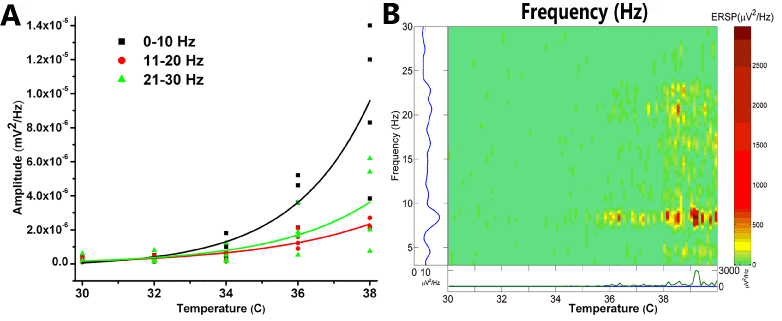
Figure 1. Recordings of population responses in PPN. A. Graph of frequencies, 0-10 Hz black squares, 11-20 Hz red circles, and 21-30 Hz green triangles, as the slices were warmed from 30°C to 38°C. Amplitude at each frequency is plotted for four different slices, with best-fit lines for each frequency range. B. Event related spectral perturbation (ERSP) of one slice showing changes in frequency (left axis) during changes in temperature (horizontal axis) and density of activity (right axis color scale) [15]. Note that, in the absence of stimulation or drug superfusion, increasing temperature led to an increase in higher frequency spontaneous activity. Significant increases in beta activity were not evident until the slice was warmed above 36°C [7,9].
Also interesting is the fact that PPN frequency patterns can vary according to arousal states. It has been shown that PPN neurons exhibit beta/gamma frequencies in vivo during active waking and REM sleep, but not during slow wave sleep [15-21]. Similarly, the presence of gamma band activity has been confirmed in the cortical EEG of the cat in vivo when the animal is active [17,22]; in the region of the PPN in primates when locomoting but not when merely standing [23]; and in the region of the PPN in humans during stepping, but not at rest [24]. Thus, there is ample evidence for gamma band activity during active waking in the PPN in vitro, in vivo, and across species, including man.
Altogether, the evidence suggests that the PPN manifests higher frequency activity (beta/gamma) when receiving sensory input or when initiating, triggering, or recruiting movement, i.e., the stimulated, active waking state. In contrast, the PPN manifests a basal firing frequency of 8-10 Hz in the absence of input, i.e., the unstimulated, quiet waking state [7-9]. This has led to the notion that ~10 Hz alpha frequency waves (occipital alpha, motor mu, and temporal tau) constitute the brain’s “idle” rhythms [25]. Of the rhythms described to date, the occipital, mu, and tau have been characterized most fully.
Occipital alpha rhythm
The occipital “alpha rhythm” is the best-known 10 Hz rhythm with a history stemming from the earliest days of the EEG. Originally described by Hans Berger, alpha waves manifested in the occipital region at 7.5 Hz to 12.5 Hz once a subject undergoing EEG recordings closed their eyes. Subsequent studies reported that in humans undergoing MEG recordings with their eyes closed exhibited a peak alpha wave frequency that is closer to 10 Hz [26].
Motor mu rhythm
The mu rhythm was first described by Gastaut [27], as a 8-12 Hz wave present over the vertex and bilaterally across the precentral motor cortex, basically at the EEG C3, Cz, and C4 electrode placements. The mu rhythm is present when the body is at rest, but is replaced when a person performs a motor action, practices a motor action, or visualizes a motor action [28]. It is also suppressed during tactile stimulation [29].
Temporal tau rhythm
Evidence for another 10 Hz rhythm in the superior and middle temporal region has been reported. The temporal rhythm is referred to as the tau rhythm. It emerges spontaneously in the auditory cortex in the absence of stimulation, but is replaced with higher frequencies following input [30].
The critical denominator of the alpha frequencies is that they emerge spontaneously in the sensorimotor areas of the brain in the absence of relevant stimuli, but shift to higher frequencies (beta/gamma) following stimulation. So why does the 10 Hz alpha frequency appear to be the “idle” mode? Metaphorically speaking, 10 Hz is the natural idle speed of the brain’s “engine” at rest, and lower speeds lead to “sputtering” in sensorimotor function (since sensorimotor stimuli cannot facilitate perceptual processing optimally at frequencies <10 Hz). Conversely, just as the engine’s idle can be increased following gas infusion (after pressing on the gas pedal), so too can the brain’s frequency (alpha) be increased (to beta/gamma) following revving (activation) of the RAS (following sensorimotor infusion). Indirect evidence from fMRI blood flow studies in the brain support the notion that alpha rhythms (the occipital and mu) are idle rhythms given that they are coincident with decreases in cerebral blood flow [28], a proxy measure for brain activity level. Also supporting the notion of resting rhythms is evidence that the vertex-recorded mu rhythms, which appear to be downstream markers of PPN function, fluctuate with the presence or absence of sensorimotor stimuli. It has been shown that the mu rhythm is present at the vertex (EEG Cz electrode location), the very area where the P50 midlatency auditory evoked potential is maximal and where blood flow is altered most significantly following PPN stimulation [31]. Finally, the vertex is precisely the location where wave changes occur in advance of voluntary movement [as demarcated by the readiness potential (RP)], an event that may reflect a shift to higher frequencies (beta/gamma) in preparation for motor events.
The readiness potential
The Readiness Potential (RP), as originally described by Kornhuber, is a negative DC shift that occurs in advance of voluntary, uncued movement. It is present at maximal amplitude at the vertex in the region of the supplementary motor cortex and precentral cortex [32,33], and precedes movement by approximately 600-800 milliseconds [32]. Interestingly, the RP manifests over the same region as the midlatency auditory evoked P50 potential and mu rhythm-a co-occurrence that is unlikely to stem from coincidence or separate phenomena. Indeed, a parsimonious explanation for this co-localization is that these markers (RP, P50, and mu) reflect a common process inherent to sensorimotor events. Given that the P50 potential is an arousal-related waveform that is elicited by sensory input [34], and is generated by PPN output that traverses the intralaminar thalamus and cortex, and that the P50 co-localizes with the mu and RP, it seems plausible that the RP is a manifestation of a functional shift in PPN firing activity (as denoted by an alpha to beta/gamma transition on EEG) in preparation for motor activity [2]. This knowledge establishes a warrant to determine whether the RP represents RAS-derived preparatory activity for movement, especially given that intense sensory stimuli that impinges on the RAS induces early latency, unintentional planned movements [35].
Physiological tremor
Voluntary movement is superimposed on a 10 Hz signal called the “physiological tremor”, a phenomenon that is well-characterized by Llinás in his book “I of the Vortex: From neurons to self” [36]. Early investigators of motor performance noted a recurrent waveform in the 8-12 Hz range that seemed to always accompany voluntary movement. That is, the 8-12 Hz wave was present in the motor cortex when limbs were supported, at rest, or maintaining static postures [37]. Later, investigators employed electromyography (EMG) along with EEG to determine how velocity affected the underlying ~10 Hz signal. Consequently, it was determined that a ~10 Hz frequency was omnipresent, irrespective of the velocity range (slow, medium, or fast) [38]. This knowledge led to the idea that voluntary movement is superimposed upon an ~10 Hz signal, a signal that is generated within the brain. Further refining this concept, Llinás conjectured that physiological tremor reflects a descending motor command from the brain and is delivered in pulsatile fashion [36]. That is, centrally derived motor commands as well as movements are inherently discontinuous in nature.
Where and by what means does an ~10 Hz signal that underlies motor movement originate? Evidence suggests the signal originates in the inferior olive (a cerebellar nucleus) and is a product of unique neurophysiogical characteristics that are characteristic to this brain region. Inferior olive neurons manifest membrane oscillations at 8-12 Hz, and fire action potentials at 1-2 Hz in phase with the peak of these oscillations [39]. Noteworthy is the fact that inferior olive cells exhibit one of the highest electrical coupling ratios (~10%) in the brain, a property that enables them to rapidly propagate a coherent 10 Hz signal to the other regions in the brain that are involved in motor control (particularly the precentral cortex). Admittedly, it is not known whether the inferior olive is the only region responsible for generating the resting rhythms that underlie voluntary movement. Notwithstanding, evidence does suggest that the physiological tremor is coherent among brain regions [40], although additional evidence in support of this hypothesis is needed.
Similar to trends seen in other brain regions, functional shifts in brain frequency (from alpha to gamma band) accompany motor stimulation. It has been shown that gamma band frequencies are present in the cortex, basal ganglia, and cerebellum following presentation of motor stimuli and these frequencies are coherent among regions [2]. For example, gamma band activity in the monkey cortex and cerebellum is coherent when performing a precision grip task [41]. Similarly, gamma band coherence exists between the basal ganglia and cortex [42,43], and the cerebellothalamic system and cortex [40] with this motor task.
Together, this bevy of evidence derived from studies on physiological tremor suggests there is a pervasive 10 Hz frequency in areas of the brain that are responsible for motor function. Moreover, this evidence suggests that a functional shift occurs (from 10 Hz alpha frequency to 40 Hz gamma frequency) within these regions with motor stimulation, a trend that is in keeping with findings from sensory investigations.
The 10 Hz frequency fulcrum
Figure 2 demonstrates the principle behind the 10 Hz rhythms (occipital alpha, mu, tau, and inferior olive) that functions as a veritable fulcrum upon which shifts to higher or lower frequencies occur. The top row contains EEG records representative of various states. Herein, the occipital alpha becomes evident in a person at rest with eyes closed, but is replaced with beta/gamma with eyes opened. A theta rhythm is present with eyes closed and drowsiness, but shifts leftward to delta with frank sleep. Finally, inferior olive rhythms are captured in the power spectrum of EMGs of voluntary movements. The normal physiological rhythm manifests pathologically as a reduced “tremor at rest” in the 6-8 Hz range, an occurrence frequently observed in Parkinson’s disease (PD). It will manifest a further reduced state as choreoathetoid writhing in the 2-4 Hz range, an occurrence that can be observed in Huntington’s disease (HD). Thus, decrements in the typical 10 Hz drive induce qualitative movement deficits at rest.
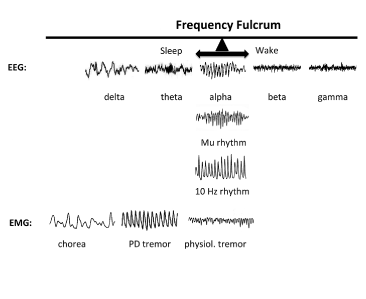
Figure 2. The 10 Hz Frequency Fulcrum. The top row shows examples of EEG recordings during various states. With eyes closed, occipital alpha is present in the EEG, but when eyes are opened the resting frequency increases to beta and gamma depending on the task. If the subject becomes drowsy, the resting frequency decrease to theta, and then to delta upon reaching frank sleep. The synchronization of low frequencies in epilepsy marks maximal coherence across very large cell ensembles. At the fulcrum are also the precentral or motor cortex mu rhythm and the inferior olive 10 Hz rhythm. The 10 Hz rhythm of physiological tremor is present in the power spectrum of EMGs of voluntary movements. If physiological tremor decreases, it results in a “tremor at rest” in the 6-8 Hz frequency, such as is manifested in Parkinson’s disease. Even slower rhythm at rest will be manifested as choreo-athetoid writhing in the 2-4 Hz range, such as is present in Huntington’s disease [2,44].
The implications of the proposal that the 10 Hz frequency facilitates transition shifts and, in so doing, is carried along with faster frequencies is far-reaching. The idea implicitly suggests that the 10 Hz frequency represents the “carrier” frequency of the brain, the very signal upon which faster frequencies are superimposed. A carrier frequency is defined as a wave that carries embedded information. For example, radio stations frequently emit carrier frequencies that carry multiple forms of information embedded in it. One of the components conveys the music of a song whereas another conveys the voice. Similarly, we regard the 10 Hz rhythm as one that facilitates the transition to higher frequencies (gamma/beta) and, then, is carried along during the process of sensorimotor processing. This trend appears to be recapitulated by theta oscillations in the hippocampus [45].
Natural frequency
There have been a number of investigations to determine whether brain circuits resonate at a natural frequency. Consequently, early studies showed that sensory stimulation (in the form of light flashes and auditory tones) induced thalamocortical resonant frequencies of ~ 10 Hz in both humans and animals [46-48]. Notwithstanding, the responses garnered reflected activation of thalamocortical circuits along with a multiplicity of other factors (peripheral receptors, intervening synapses, changes in attention, and sensory gating). To circumvent this issue, later studies deployed more direct perturbations of the thalamocortical circuits [49]. Similarly, these authors used transcranial magnetic stimuli to directly perturb cortical regions and noted a return to a seemingly natural resonant frequency following removal of the stimulus [50].
In parallel investigations of natural frequencies in vitro, we subjected sagittal slices of rodent PPN to neurochemical and electrical stimulation and then recorded changes in resonance using microelectrodes to record population responses. We noted transmitter specific effects wherein kainic acid, which is known to modulate REM sleep [20], induced a time-dependent, step-wise increase from alpha, to theta, and to gamma frequency [7]; NMDA, which is known to modulate waking [51], induced increases in every frequency range, including gamma [7]; and carbachol, which is known to initiate and maintain PPN activity [8], induced alpha and gamma frequencies [7]. Particularly noteworthy was the fact that every transmitter increased the resonance of alpha frequency [7].
Subsequently, we subjected PPN slices to carbachol treatment and trains of electrical stimuli at 1, 10, and 40 Hz. We found that application of a 1 Hz train induced resonance across all bands whereas application of a 10 Hz train induced resonance in the beta range, with the latter indicating an amplification of response. Finally, we noted that trains of 40 Hz induced impressive resonance in the gamma range. These results were interpreted to suggest that alpha frequency represents the natural “resting” frequency of the PPN that occurs in the absence of exogenous stimuli, but that maximal coherence in PPN cells occurs at gamma band frequency. The unique cellular characteristics of PPN cells likely give rise to their ability to generate the gamma band activity that is propagated to the intralaminar thalamus [10,12].
PPN cells manifest a combination of N- and/or P/Q-type calcium channels that permit maximal firing after depolarization [8,9,14] at beta/gamma frequencies [7-14,52-54]. Figure 3 illustrates the method used as square steps of depolarizing currents were unable to induce high frequency membrane oscillations, probably due to the presence of potassium channels activated by rapid depolarization. On the other hand, depolarizing ramps of the same amplitude were able to drive the membrane potential to sufficiently high levels to elicit high frequency (beta/gamma) membrane oscillations [9,13,14].
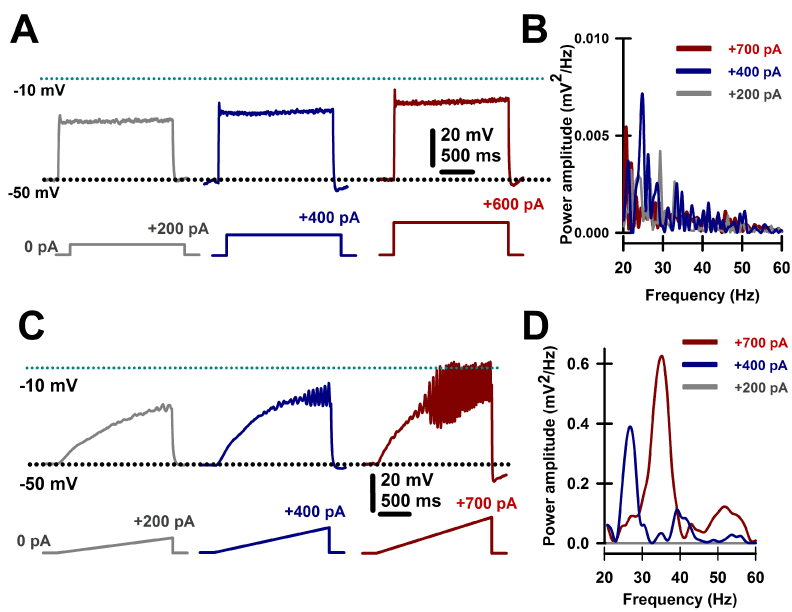
Figure 3. Intrinsic membrane oscillations in PPN neurons. A. Recordings were performed in the presence intracellular high potassium solution and extracellular fast synaptic blockers and sodium channel blockers to study only intrinsic membrane properties. Square steps of increasing current levels depolarized the membrane but failed to induce clear high frequency oscillations. B. Overlapping power spectra amplitudes for oscillations obtained using steps shown in A. Power spectra were obtained using a Hamming window function after 20-60 Hz bandpass filtering oscillations generated by the depolarizing steps. Note the absence of beta/gamma frequency membrane oscillations. C. Recordings performed as in A, except using depolarizing ramps of similar currents. Ramps were able to sufficiently depolarize the membrane to induce robust oscillations, later confirmed to be due to high threshold, voltage-dependent calcium channels. D. Overlapping power spectra amplitudes for oscillations obtained using ramps shown in C. Power spectra were obtained using a Hamming window function after 20-60 Hz bandpass filtering oscillations generated by the depolarizing ramps. Note the presence of beta/gamma frequency membrane oscillations.
We conjectured that unique combinations of channel types in different groups of cells might contribute to the oscillations present in different states and, thus, characterized their distribution. We found three distinct groups of cells: those containing only N-type channels (30%); those containing only P/Q-type channels (20%), and those containing both N- and P/Q-type channels (50%) [14]. These distinctions were evident after we showed that oscillatory activity in one group of cells was abolished with application of an N-type channel blocker (ω-conotoxin-GVIA) whereas the oscillatory activity of another group was abolished with administration of a P-type channel blocker (ω-agatoxin-IVA). Finally, the remaining group of cells required the application of both N- and P-type channel blockers to abolish oscillatory activity [14].
We extended these studies by hypothesizing a “waking” pathway that was mediated by P/Q channels and CaMKII activity along with a “REM sleep” pathway mediated by N-type channels and cAMP/PK activity. This supposition was premised upon studies demonstrating that 1) CaMKII is essential for P/Q-type channel function [55], 2) protein kinase C (PKC) is essential for N-type channel activity [56], 3) waking and REM sleep states are differentially activated by NMDA vs KA receptors [19,51,57], and 4) gamma band activity is fundamentally different during waking than during REM sleep [14]. That is, we proposed a new cell type classification wherein some PPN neurons fire only during REM sleep (“REM-on”, N-type only), only during waking (“Wake-on”, P/Q-type only), or during both waking and REM sleep (“Wake/REM-on”, N-type + P/Q-type) [14]. Figure 4 graphically illustrates the distribution of PPN cells containing N-only, P/Q-only and N+P/Q calcium channels in proportion to electrophysiological type I, II, or III. Since type I cells are non-cholinergic, type II cells are 2/3 cholinergic, and type III cells are 1/3 cholinergic, it is likely that all electrophysiological types and all transmitter types in the PPN (cholinergic, glutamatergic, GABAergic) manifest one or both calcium channels [2].
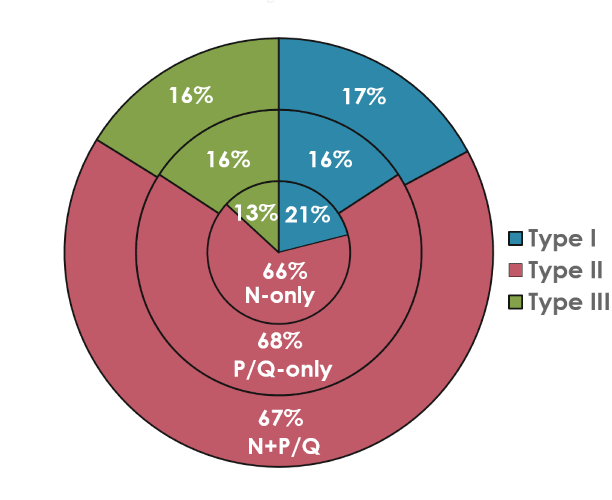
Figure 4. Pie chart of the proportion of PPN neurons with N- vs P/Q-type calcium channels by cell type. Percentage of PPN cells manifesting both N+P/Q-type calcium channels (outer ring), PPN cells manifesting only N-type channels (middle ring), and those manifesting only p/Q-type channels (inner ring). The proportion of type I cells is in brown (13-16%), of type II cells is in purple (66-68%), and of type III cells (13-16%). These results suggest that all three electrophysiological types, and probably all three transmitter types (type I cells are non-cholinergic, type II cells are 2/3 cholinergic, and type III cells are 1/3 cholinergic) bear all three calcium channels types (N only, P/Q only, both N+P/Q) [9,13,14].
Clinical implications
A host of neurological and psychiatric disorders have a common characteristic: interruptions in alpha and gamma band frequency. Decrements in alpha band manifest as problems with the induction or maintenance of high frequency activity. Disruptions in gamma band manifest as attentional, cognitive, perceptual, and mnemonic deficits. Recognition of this prompted us to propose a model wherein the pervasive 10 Hz wave functions as a Frequency Fulcrum, a functional pivot between sleep and waking states. The model provides an objective tool that can be used to assess dynamic wake and sleep states in both health and disease, particularly given that EEG records are readily obtainable from persons affected by neurological and psychiatric conditions. By corollary, this model provides an objective means to assess the effects of therapeutic interventions that aim to normalize sleep/wake function. Manipulations that restore the fulcrum to more normal function can be regarded as one that produces positive health effects, whereas those that skew the spectrum towards an extreme can be considered as producing ill effects.
Preconscious awareness
Preconscious awareness refers to states and processes that originate subcortically and remain below the level of consciousness [58], a necessary semantic that is not functional in nature. Indeed, preconscious awareness is inextricably melded with consciousness and its correlates, ultimately forming the foundational “context” upon which conscious “content” is superimposed. At the crux of this process is persistent RAS activation that generates and maintains the gamma band frequencies [2,53,54].
During waking, RAS activation is enhanced by sensory volleys from the ambient environment, stimuli that increases the level of excitability in cells [2]. This means, mechanistically speaking, that afferent sensory information arising from collateral activation of the RAS triggers dendritic oscillations in PPN neurons. Figure 5 illustrates the putative pathways involved. Specifically, the presence of sensory inputs to dendrites with P/Q- and N-type Ca2+ channels induce gamma band oscillations and, in turn, influence the overall frequency of PPN firing. Then, PPN output projects superiorly to the intralaminar thalamus, especially the parafascicular nucleus (Pf), to induce dendritic oscillations at gamma frequency via P/Q- and N-type Ca2+ channels [10,44]. The projections of Pf cells, particularly those traveling to upper cortical layers where the nonspecific thalamic inputs terminate, induce gamma frequencies in cortical cells that resonate back and forth, creating the context for awareness. In parallel, corticothalamic resonance between specific thalamic relay nuclei and layer IV creates the content of sensory experience. Coincident firing of these two pathways represents a putative mechanism for sensory perception and motricity [26]. Noteworthy is the fact that gamma band is easily maintained in cortical, hippocampal, and cerebellar cells once it has been generated [59].
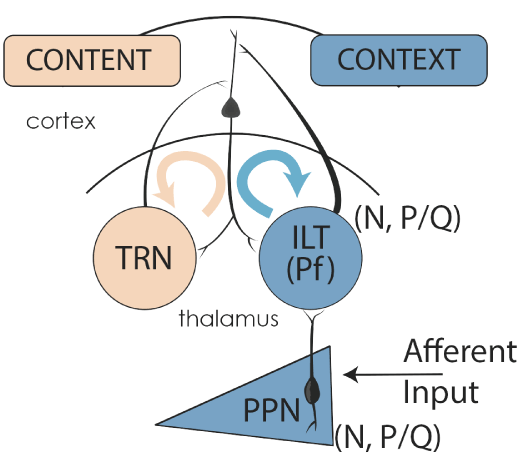
Figure 5. Gamma band activity in the RAS and ascending targets. Afferent sensory information impinging on dendrites with high threshold calcium channels induces gamma band activity in PPN neurons. PPN output during REM sleep (N+P/Q and N-only cells) activate SubCD neurons with sodium-dependent subthreshold oscillations (STO) (Simon et al 2011, 2012), which in turn travel to the hippocampus and descending targets. PPN output during waking (N+P/Q and P/Q-only cells) activate the dendrites of parafascicular (Pf) cells in the intralaminar thalamus (ILT) that also bear high threshold calcium channels in the dendrites [12]. Gamma band activity from the “non-specific” ILT travels to upper layers of the cortex to supply the “CONTEXT” of sensory input, while “specific” thalamic relay neurons (TRN) send information to layer IV of the cortex to provide the “CONTENT” of sensory experience. The resulting thalamocortical oscillations based on coincident firing provides conscious perception [26,36].
Based on the fact that every neuron in each of the major nuclei that modulates waking and REM sleep manifests gamma band activity, we characterized the RAS as a “gamma-making machine” [2,44,60]. This machine works in concert with other regions of the central nervous system that exhibit gamma band activity-such as the cortex, thalamus, cerebellum, basal ganglia, hippocampus, and RAS- to facilitate coherence among regions. That is, these gamma band generators are not isolated but correlated, and in some cases subcortical oscillations precede cortical oscillations. Based on the presence of electrical coupling, intrinsic membrane properties, and unique circuitry that is capable of generating and maintaining gamma band activity, we proposed a novel role for the RAS: supporting a contextual background state necessary for reliably assessing and interacting with the world [2,44,59,60]. Also, we suggested that gamma band activity is generated in the PPN and propagated via electrical coupling, a characteristic endemic to the region and one that provides a stable activation state. Then, we identified intracellular mechanisms (intrinsic membrane properties) that enable the generation and maintenance of gamma band frequencies for prolonged periods. Finally, we proposed that sensory input induces gamma band activity in the RAS and participates in preconscious awareness (Figure 4).
William James proposed that the “stream of consciousness” is “a river flowing forever through a man’s conscious waking hours” [61]. This dynamic stream persistently infiltrates the essence of our mind, yet we often fail to pay it heed, letting much sensorimotor information go unnoticed. Once beckoned into awareness, we can actively pay tribute to a piece of sensorimotor information: we can become fully “conscious” of it [58]. At the wellspring of this pre-conscious process is the RAS, a phylogenetically conserved area of the brain that is inundated by the continuous flow of endogenous and exogenous information. By traversing RAS structures and projection areas, this information modulates wake-sleep cycles, the startle response, and fight vs flight responses (e.g., changes in muscle tone and locomotion). Accordingly, we speculate that activation of the RAS during waking induces coherent activity (through electrically coupled cells) and high-frequency oscillations (through N- and P/Q-type Ca2+ channel and subthreshold oscillations) to sustain gamma activity (through activation of G-proteins) and support a persistent, reliable state needed to integrate the sensorimotor process of preconscious awareness that is instrumental for interaction with our world.
Acknowledgments
This work was supported by NIH award R01 NS020246, and by core facilities of the Center for Translational Neuroscience supported by NIH award P20 GM103425 and P30 GM110702 to Dr. Garcia-Rill. In addition, BID 1728 OC.AR. PICT 2012-1769 and UBACYT 2014-2017 #20120130101305BA grants from FONCYT-Agencia Nacional de Promoción Científica y Tecnológica (Argentina) supports Dr. Francisco J. Urbano.
Conflict of interest
None of the authors have a financial interest or conflict.
References
- Llinás RR, Paré D (1991) Of dreaming and wakefulness. Neuroscience 44: 521-535. [Crossref]
- Garcia-Rill E (2015) Waking and the Reticular Activating System. New York Academic Press, USA, pp. 330.
- Tsang EW, Hamani C, Moro E, Mazzella F, Poon YY, et al. (2010) Involvement of the human pedunculopontine nucleus region in voluntary movements. Neurology 75: 950-959. [Crossref]
- Tattersall TL, Stra2021 Copyright OAT. All rights reservn P, et al. (2014) Imagined gait modulates neuronal network dynamics in the human pedunculopontine nucleus. Nat Neurosci 17: 449-454. [Crossref]
- Thevathasan W, Cole MH, Graepel CL, Hyam JA, Jenkinson N, et al. (2012) A spatiotemporal analysis of gait freezing and the impact of pedunculopontine nucleus stimulation. Brain 135: 1446-1454. [Crossref]
- Mena-Segovia J, Sims HM, Magill PJ, Bolam JP (2008) Cholinergic brainstem neurons modulate cortical gamma activity during slow oscillations. J Physiol 586: 2947-2960. [Crossref]
- Simon C, Kezunovic N, Ye M, Hyde J, Hayar A, et al. (2010) Gamma band unit activity and population responses in the pedunculopontine nucleus. J Neurophysiol 104: 463-474. [Crossref]
- Kezunovic N, Hyde J, Goitia B, Bisagno V, Urbano FJ, et al. (2013) Muscarinic modulation of high frequency oscillations in pedunculopontine neurons. Front Neurol 4: 176. [Crossref]
- Kezunovic N, Urbano FJ, Simon C, Hyde J, Smith K, et al. (2011) Mechanism behind gamma band activity in the pedunculopontine nucleus. Eur J Neurosci 34: 404-415. [Crossref]
- Kezunovic N, Hyde J, Simon C, Urbano FJ, Williams DK, et al. (2012) Gamma band activity in the developing parafascicular nucleus. J Neurophysiol 107: 772-784. [Crossref]
- Hyde J1, Kezunovic N, Urbano FJ, Garcia-Rill E (2013) Spatiotemporal properties of high-speed calcium oscillations in the pedunculopontine nucleus. J Appl Physiol (1985) 115: 1402-1414. [Crossref]
- Hyde J, Kezunovic N, Urbano FJ, Garcia-Rill E (2013) Visualization of fast calcium oscillations in the parafascicular nucleus. Pflugers Arch 465: 1327-1340. [Crossref]
- D'Onofrio S, Kezunovic N, Hyde JR, Luster B, Messias E, et al. (2015) Modulation of gamma oscillations in the pedunculopontine nucleus by neuronal calcium sensor protein-1: relevance to schizophrenia and bipolar disorder. J Neurophysiol 113: 709-719. [Crossref]
- Luster B, D'Onofrio S, Urbano F, Garcia-Rill E (2015) High-threshold Ca2+ channels behind gamma band activity in the pedunculopontine nucleus (PPN). Physiol Rep 3. [Crossref]
- Delorme A, Makeig S (2004) EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134: 9-21. [Crossref]
- Sakai K, el Mansari M, Jouvet M (1990) Inhibition by carbachol microinjections of presumptive cholinergic PGO-on neurons in freely moving cats. Brain Res 527: 213-223. [Crossref]
- Steriade M, Paré D, Datta S, Oakson G, Curró Dossi R (1990) Different cellular types in mesopontine cholinergic nuclei related to ponto-geniculo-occipital waves. J Neurosci 10: 2560-2579. [Crossref]
- Kayama Y, Ohta M, Jodo E (1992) Firing of 'possibly' cholinergic neurons in the rat laterodorsal tegmental nucleus during sleep and wakefulness. Brain Res 569: 210-220. [Crossref]
- Datta S, Siwek DF (2002) Single cell activity patterns of pedunculopontine tegmentum neurons across the sleep-wake cycle in the freely moving rats. J Neurosci Res 70: 611-621. [Crossref]
- Datta S, Siwek DF, Stack EC (2009) Identification of cholinergic and non-cholinergic neurons in the pons expressing phosphorylated cyclic adenosine monophosphate response element-binding protein as a function of rapid eye movement sleep. Neurosci 163: 397-414. [Crossref]
- Boucetta S, Cissé Y, Mainville L, Morales M, Jones BE (2014) Discharge profiles across the sleep-waking cycle of identified cholinergic, GABAergic, and glutamatergic neurons in the pontomesencephalic tegmentum of the rat. J Neurosci 34: 4708-4727. [Crossref]
- Steriade M, Dossi RC, Paré D, Oakson G (1991) Fast oscillations (20-40 Hz) in thalamocortical systems and their potentiation by mesopontine cholinergic nuclei in the cat. Proc Natl Acad Sci U S A 88: 4396-4400. [Crossref]
- Goetz L, Piallat B, Mathieu H, David O, Chabardes S (2016) On the role of the pedunculopontine nucleus and mesencephalic reticular formation in locomotion and waking state in non-human primates. J Neural Transm, in press.
- Fraix V, Bastin J, David O, Goetz L, Ferraye M, et al. (2013) Pedunculopontine nucleus area oscillations during stance, stepping and freezing in Parkinson's disease. PLoS One 8: e83919. [Crossref]
- Pfurtscheller G (1992) Event-related synchronization (ERS): an electrophysiological correlate of cortical areas at rest. Electroencephalogr Clin Neurophysiol 83: 62-69. [Crossref]
- Llinás RR, Ribary U, Jeanmonod D, Cancro R, Kronberg E, et al. (2001) Thalamocortical dysrhythmia I Functional and imaging aspects. Thalamus Rel Syst 1: 237-244.
- Gastaut H, Dongier M, Courtois G (1954) On the significance of “wicket rhythms” (“rhythmes en arceau”) in psychosomatic medicine. Electroenceph Clin Neurophysiol 6: 687-688.
- Ritter P, Moosmann M, Villringer A (2009) Rolandic alpha and beta EEG rhythms' strengths are inversely related to fMRI-BOLD signal in primary somatosensory and motor cortex. Hum Brain Mapp 30: 1168-1187. [Crossref]
- Cheyne D, Gaetz W, Garnero L, Lachaux JP, Ducorps A, et al. (2003) Neuromagnetic imaging of cortical oscillations accompanying tactile stimulation. Brain Res Cogn Brain Res 17: 599-611. [Crossref]
- Lehtelä L, Salmelin R, Hari R (1997) Evidence for reactive magnetic 10-Hz rhythm in the human auditory cortex. Neurosci Lett 222: 111-114. [Crossref]
- Ballanger B, Lozano AM, Moro E, van Eimeren T, Hamani C, et al. (2009) Cerebral blood flow changes induced by pedunculopontine nucleus stimulation in patients with advanced Parkinson's disease: a [(15)O] H2O PET study. Hum Brain Mapp 30: 3901-3909. [Crossref]
- kornhuber Hh, Deecke L (1965) Changes in the brain potential in voluntary movements and passive movements in man: readiness potential and reafferent potentials. Pflugers Arch Gesamte Physiol Menschen Tiere 284: 1-17. [Crossref]
- Deecke L, Grözinger B, Kornhuber HH (1976) Voluntary finger movement in man: cerebral potentials and theory. Biol Cybern 23: 99-119. [Crossref]
- Garcia-Rill E, Skinner RD (2001) The sleep state-dependent P50 midlatency auditory evoked potential. Hanley & Belfus, Philadelphia, USA, pp. 697-704.
- Mackinnon CD (2013) New strides in wearable sensor technology. Mov Disord 28: 1025-1026. [Crossref]
- Llinás RR (2001) I of the Vortex, from neurons to self. MA. MIT Cambridge, USA.
- Marsden CD, Rothwell JC, Day BL (1984) The use of peripheral feedback in the control of movement. Trends Neurosci 7: 253-257.
- Vallbo AB, Wessberg J (1993) Organization of motor output in slow finger movements in man. J Physiol 469: 673-691. [Crossref]
- Llinás RR (1981) Microphysiology of the cerebellum. Bathesda, MD. Amer Physiol Soc, pp. 831-976.
- Timofeev I, Steriade M (1997) Fast (mainly 30-100 Hz) oscillations in the cat cerebellothalamic pathway and their synchronization with cortical potentials. J Physiol 504 : 153-168. [Crossref]
- Soteropoulos DS, Baker SN (2006) Cortico-cerebellar coherence during a precision grip task in the monkey. J Neurophysiol 95: 1194-1206. [Crossref]
- Lalo E, Thobois S, Sharott A, Polo G, Mertens P, et al. (2008) Patterns of bidirectional communication between cortex and basal ganglia during movement in patients with Parkinson disease. J Neurosci 28: 3008-3016. [Crossref]
- Litvak V, Eusebio A, Jha A, Oostenveld R, Barnes G, et al. (2012) Movement-related changes in local and long-range synchronization in Parkinson's disease revealed by simultaneous magnetoencephalography and intracranial recordings. J Neurosci 32: 10541-10553. [Crossref]
- Garcia-Rill E, Kezunovic N, Hyde J, Simon C, Beck P, et al. (2013) Coherence and frequency in the reticular activating system (RAS). Sleep Med Rev 17: 227-238. [Crossref]
- Kalauzi A, Spasic S, Petrovic J, Ciric J, Saponjic J (2012) Cortico-pontine theta carrier frequency phase shift across sleep/wake states following monoaminergic lesion in rat. Gen Physiol Biophys 31: 163-171. [Crossref]
- Narici L, Romani GL (1989) Neuromagnetic investigation of synchronized spontaneous activity. Brain Topogr 2: 19-30. [Crossref]
- Rager G, Singer W (1998) The response of cat visual cortex to flicker stimuli of variable frequency. Eur J Neurosci 10: 1856-1877. [Crossref]
- Herrmann CS (2001) Human EEG responses to 1-100 Hz flicker: resonance phenomena in visual cortex and their potential correlation to cognitive phenomena. Exp Brain Res 137: 346-353. [Crossref]
- Rosanova M, Casali A, Bellina V, Resta F, Mariotti M, et al. (2009) Natural frequencies of human corticothalamic circuits. J Neurosci 29: 7679-7685. [Crossref]
- Garcia-Rill E, Moran K, Garcia J, Findley WM, Walton K, et al. (2008) Magnetic sources of the M50 response are localized to frontal cortex. Clin Neurophysiol 119: 388-398. [Crossref]
- Datta S, Siwek DF (1997) Excitation of the brain stem pedunculopontine tegmentum cholinergic cells induces wakefulness and REM sleep. J Neurophysiol 77: 2975-2988. [Crossref]
- Kezunovic N, Simon C, Hyde J, Smith K, Beck P, et al. (2010) Arousal from slices to humans: Translational studies on sleep-wake control. Transl Neurosci 1: 9-15. [Crossref]
- Garcia-Rill E, Luster B, D'Onofrio S, Mahaffey S, Bisagno V, et al. (2015) Implications of gamma band activity in the pedunculopontine nucleus. J Neural Transm (Vienna). [Crossref]
- Garcia-Rill E, Luster B, Mahaffey S, MacNicol M, Hyde JR, et al. (2015) Pedunculopontine Gamma Band Activity and Development. Brain Sci 5: 546-567. [Crossref]
- Jiang X, Lautermilch NJ, Watari H, Westenbroek RE, Scheuer T, et al. (2008) Modulation of CaV2.1 channels by Ca2+/calmodulin-dependent protein kinase II bound to the C-terminal domain. Proc Natl Acad Sci U S A 105: 341-346. [Crossref]
- Stea A, Soong TW, Snutch TP (1995) Determinants of PKC-dependent modulation of a family of neuronal calcium channels. Neuron 15: 929-940. [Crossref]
- Datta S, Patterson EH, Spoley EE (2001) Excitation of the pedunculopontine tegmental NMDA receptors induces wakefulness and cortical activation in the rat. J Neurosci Res 66: 109-116. [Crossref]
- Civin M, Lombardi KL (1990) The preconscious and potential space. Psychoanal Rev 77: 573-585. [Crossref]
- Urbano FJ, D'Onofrio SM, Luster BR, Beck PB, Hyde JR, et al. (2014) Pedunculopontine Nucleus Gamma Band Activity-Preconscious Awareness, Waking, and REM Sleep. Front Neurol 5: 210. [Crossref]
- Garcia-Rill E, Kezunovic N, D'Onofrio S, Luster B, Hyde J, et al. (2014) Gamma band activity in the RAS-intracellular mechanisms. Exp Brain Res 232: 1509-1522. [Crossref]
- James W (1890) The Principles of Psychology. Volume 1:225.





