Endogenous creatine is created in a stable, slightly alkaline, environment. It is reasonably efficacious in delivering ATP to its target in the body. Manufactured creatine is introduced orally into an acidic environment. A percentage is degraded in the stomach, or as a result of acidophilic bacterial exposure. In either case, a certain amount of orally ingested creatine never becomes available for ATP transport. Creatine supplementation, in and of itself, has been shown to influence endothelial permeability and cell surface reactivity to a modest degree, potentially interfering/blocking an inflammatory stimulus. In vitro endothelial cell adhesion experiments demonstrate that, as creatine concentrations increase, endothelial cell surface adhesion and permeability were both modified.
Creatine does not cross the plasma membrane very well. Roughly 95% of creatine is released in close proximity to the muscle tissue and transported into the cell via a limited capacity creatine-transporter system.
Recent reports have shown that an artificially created alkaline environment can influence cell membrane permeability and cell behavior. The alkalinity of soda has shown anti-proliferative effects on tumor cell lines. The ability of alkaline buffered creatine to produce micro-environmental remodeling, influence cell membrane behavior and impact cisplatin-mediated toxicity, are also examined in this paper.
Kre-Alkalyn®, micro-alkaline environment, tumor cells, cisplatin, cellular toxicity
Creatine (N-(aminoiminomethyl-N-methyl glycine) is an important amino acid based metabolite produced in the kidneys, liver and pancreas and used by the muscle in the production of energy [1-3]. Since its first description in 1832, its role in muscle metabolism has been touted. Creatine can be obtained from meat and fish products, or through de novo synthesis. Metabolic creatine synthesis occurs via two enzymatic reactions: L-arginine, glycine amidinotransferase (AGAT) transfers an amidino group from arginine to glycine to produce guanidinoacetate (GAA) and ornithine and second, guanidinoacetate methyltransferase (GAMT) transfers a methyl group from S-adenosylmethionine to GAA to form creatine and S-adenosyl homocysteine [3].
Synthetic creatine degradation is not dependent on concentration but on pH. Several reports have noted that creatine degradation is a function of pH, as a result of an increased rate of intramolecular cyclization [2,4,5]. Based on GC-MS determination, under normal physiological conditions, it is estimated that 10% of ingested creatine is converted to creatinine and roughly 78% of ingested creatine is taken up in vivo [6]. Creatine has very few side effects, which makes it an ideal supplement for metabolic experimentation [7,8].
Creatine associates with creatine kinase to produce ATP for energy production. In the phosphorylated form, it is used in energy metabolism to transfer phosphate from ADP to ATP [3]. A substantial portion of free or bound creatine and creatine phosphate are found in mammalian skeletal muscle tissue. Approximately 60% of the creatine in the body is found in the phosphorylated form [9]. Creatinine is the major endproduct of creatine. This ‘spent fuel’ byproduct circulates freely in the blood and is excreted in the urine [1].
Creatine is pivotally important in delaying ATP depletion during anoxia or ischemia through the creatine-phosphocreatine system [10,11]. Today, many athletes take a synthetic creatine powder supplement for performance enhancement. Studies focused on creatine supplementation primarily report a positive correlation between creatine uptake and exercise performance [9,12,13]. Creatine supplementation has been shown to be capable of reducing decrement of lower-limb power in elite soccer players during training [14,15]. Recently studies have been conducted to assess the potential for this product to be used in delaying the onset of age-related diseases including stroke, Alzheimer’s disease, chronic heart disease and chronic muscle wasting diseases such as amyotrophic lateral sclerosis [7,16]. A number of deficiencies in the metabolic process – including, Arginine-glycine amidinotransferase deficiency, an inherited disorder that primarily affects the brain. Inborn errors in either of the enzymes GAA and GAMT lead to serious disease states involving neurological impairments and provide a potential pharmacological role for creatine supplementation. Supplemental creatine has been effective in blunting the appearance of the resulting developmental disabilities [3]. In these instances, anything that aides creatine’s entry into the cell would increase the effectiveness of the treatment.
Creatine does not cross the plasma membrane very well on its own. Roughly 95% of creatine is released in close proximity to the muscle tissue and transported into the cell via a limited capacity creatine-transporter system [1,7]. Studies have shown that dietary creatine has relatively poor absorptive properties with a maximum of 2.2 mM of creatine in the blood 2.5 hours after a single oral dose of 20 grams [17]. One of the objectives of ‘designer creatine’ is to increase its absorptive properties. A number of attempts have been made to alter the properties of synthetic creatine in order to stimulate the movement of creatine into the cells. The addition of proteins, carbohydrates, and lipophilic agents, removal of water (anhydrous creatine), creation of salts of creatine: creatine citrate, creatine phosphate, creatine ethyl ester and magnesium creatine have been attempted [7,9,10,17]. Despite these modifications, there is little evidence that these newer forms cross the cell membrane any more efficiently [2,18]. As such, supplemental creatine delivery, directly to the interior of the muscle cell, remains a technical hurdle in need of a novel approach.
Creatine supplementation, in and if itself, has been shown to influence endothelial permeability and cell surface reactivity to a modest degree, potentially interfering/blocking an inflammatory stimulus. In vitro endothelial cell adhesion experiments demonstrated that, as creatine concentrations increased, endothelial cell surface adhesion and permeability were both modified. Supplementation successfully suppressed neutrophil adhesion to endothelial cells, as well as endothelial permeability induced by serotonin and water, and inhibited the expressions of inter-cellular adhesion molecule 1 (ICAM-1) and E-selectin on the endothelial cell membrane [19]. A number of studies have suggested that existing modifications to creatine monohydrate, including its stored alkalinity, do not affect certain parameters of creatine bioavailability [4,18]; alkalinity may, never the less, have other potential utilities.
Alkaline buffered supplements have been shown to have an anti-proliferative effect in human tumor cells more so than non-buffered supplements [20], and may be effective in the treatment of juvenile arthritis [21]. Extracellular alterations in pH around the immediate cell membrane, especially in neoplastic cell models, have been shown capable of affecting the behavior of cell membrane/surface receptors. In a neoplastic model, micro-environment near the cell’s surface is usually pH 6.5 or lower due to fermentation metabolism [22], and poor perfusion [23]. It is believed that a lower pH environment encourages invasive tumor growth in primary and metastatic cancers through a form of acid-induced micro-environmental remodeling [22-25].When the pH environment was artificially increased through the use of sodium bicarbonate (NaHCO3), cellular behavior was modified, tumor growth and invasiveness was reduced [25].
In addition, an alkaline environment is stressful for bacteria such as Escherichia coli; and high pH, is usually accompanied by a loss in viability [26,27]. Spore forming contaminants such as molds and clostridia, found in herbal products, are suppressed in alkaline environments. Other studies have suggested that one of the most common microbial contaminants in food supplements – Staphylococcus aureus [28], has a significantly reduced growth rate in alkaline environments. So alkalization, on a micro-environment scale, appears to be impacting many, as yet unknown, cellular membrane functions.
Test materials and controls
In this study a commercial creatine monohydrate powder buffered with sodium carbonate (Na2CO3) to a pH of 12 was utilized (Kre-Alkalyn® (6 batches) - All American Pharmaceutical, Billings, MT). Other generic creatines including creatine monohydrate, creatine effervescent and creatine monohydrate fruit flavored (6 samples of each) were purchased at retail food supplement store to represent readily available creatine agents. For pH analysis, liquid creatine mixes were added to a glass curette and placed on a BuchiNIRFlex N-500 FTNIR testing apparatus. Measurements and data were recorded and comparison was made for these 4 different types of creatines, using 6 lots of each.
Cell lines and treatment
.Muscle cells (RD) or chondrocytes (SW1353) were sub-cultured in a 2.5% fetal calf serum to represent nutrient deficiency. Cells were then exposed to either culture medium (controls) or 0.5 mmol conventional creatine or buffered creatine (Kre-Alkalyn®) for various time points. Cells were detached by trypsinization, washed three times in PBS and counted. The protein content in each sample was determined using the Lowry method. Exponentially growing 293T cells were plated in 96-well microplates and after a 24 h adaptation period, were exposed to cisplatin (at 5, 25 or 50 μmol/L, alone or in combination with 0.2 or 1 mmol/L creatine (conventional or buffered). Following a 72 h continuous exposure, the cellular viability was assessed using the MTT-dye reduction assay, as described by Mosmann [29], with minor modifications [20,30,31]. Data shown in Figures 1 and 2 are representative of two independent experiments done in triplicates. Date presented as mean /standard deviation values where it was applicable.
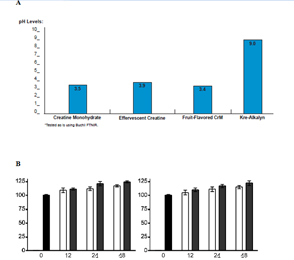
Figure 1. A: pH Analysis of Creatine Samples, B: Protein Synthesis (%)
A). pH levels for creatine monohydrate, effervescent creatine, fruit-flavored creatine, and alkalized creatine (Kre-Alkalyn®) sufficiently indicate a significant buffering capacity is maintained when supplements are first dissolved in water and then added to an acidic solution (pH 3, hydrochloric acid) for 15 minutes. (Y-axis: pH Levels, X-axis: Creatine Compounds)
B). A monolayer of muscle cells (RD) or chondrocytes (SW1353) were sub-cultured in a deficient medium containing only 2.5 % fetal calf serum. Cells were then exposed to either culture medium (controls) or 0.5 mmol of conventional creatine (white bars) or buffered creatine, Kre-Alkalyn® (black bars) for 12, 24, and 48 hrs and examined for protein synthesis. Buffered creatine, Kre-Alkalyn® led to similar, but slightly increased, protein synthesis compared to the control creatine for12, 24, and 48 hrs. (Y-axis: Protein Synthesis (%), X-axis: Hrs)
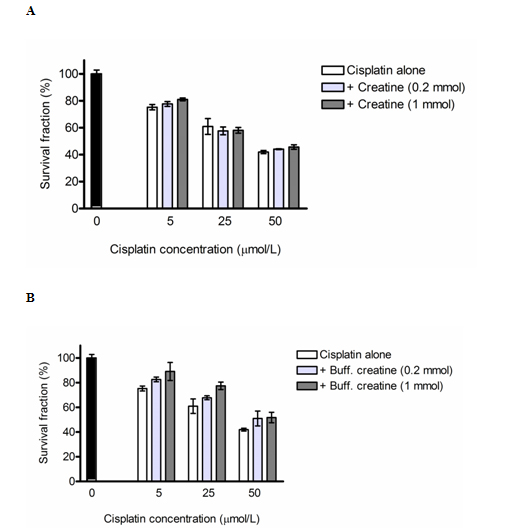
Figure 2. A: Cytotoprotective Effects of Creatine, B:
A). Cytoprotective effects of conventional creatine against cisplatin-induced cytotoxicity in 293T human kidney cells, as assessed by the MTT-dye reduction assay after 72 h incubation. Each column represents the arithmetic mean ± sd (n=6). {Y-axis: Survival Fraction (%), X-axis: Cisplatin Concentration (µmol/L = µM)}
B). Cytoprotective effects of the buffered creatine formulation (Kre-Alkalyn®) against cisplatin-induced cytotoxicity in 293T human kidney cells, as assessed by the MTT-dye reduction assay after 72 h incubation. Each column represents the arithmetic mean ± sd (n=6). {Y-axis: Survival Fraction (%), X-axis: Cisplatin Concentration (µmol/L = µM)}
Molecular stability
In order to observe the impact alkalinity had on preventing degradation, and subsequent cyclization of creatine monohydrate to creatinine, a buffered creatine (Kre-Alkalyn®) and non-buffered creatines, were exposed to an acidic environment (pH 3, hydrochloric acid) for a period of 15 minutes then tested using FTNIR technology. One and a half grams of eighteen commercially available generic creatines from three different type (6 of each), and six batches of alkaline buffered creatine (Kre-Alkalyn®), were mixed with water and each exposed to low pH solution. The final pH of the non-buffered creatine was four (pH 4). The buffered creatine – Kre-Alkalyn® remained at pH 9, and displayed an increased stability profile, whereas all other creatines tested underwent degradation (Figure 1A).
Protein synthesis
Both conventional creatine and buffered creatine were capable of increasing protein synthesis in human muscle and cartilage cells when compared to un-supplemented cultures similar to previous studies [18]. An established monolayer culture of muscle cells (RD) or chondrocytes (SW1353) were sub-cultured in a deficient medium containing only 2.5% fetal calf serum, instead of 10% which is suggested for optimal cell growth and maintenance in-vitro. Cells were then exposed to either culture medium (controls) or 0.5 mmol conventional creatine or buffered creatine. After 12 hrs, 24 hrs or 48 hrs exposure, the cells were detached by trypsinization, washed three times in PBS to remove residual protein from the culture medium, and counted. The protein content in each sample was determined using the Lowry method. The results were calculated as mgs of protein/106 cells, and expressed as a percentage of the untreated control (Figure 1B).
Cytoprotective effect if alkalization in the presence of cisplatin
The cytoprotective potential of both conventional and Kre-Alkalyn® buffered formulations was tested in a comparative fashion using a model of cisplatin-induced cytotoxicity. Cisplatin is nephrotoxic drug and the mechanisms underlying this effect are complex and involve free radical generation, oxidative and nitrosative stress, disruption of calcium homeostasis, adduct formation and bioactivation upon binding to glutathione giving rise to toxic reactive thiols [32].
Cisplatin induced a strong, concentration-dependent decrease of the 293T cellular survival, whereby the cell viability was reduced by 25% at 5 μM, reduced by 40% at 25 μM, and 55% at 50 μM when compared to untreated control (Figure 2A and 2B).
The co-administration of a non-stabilized creatine solution was consistent with marginal protective effects, which were generally more pronounced at the higher level of creatine (1 mmol/L). Statistically significant decrease of cisplatin cytotoxicity was encountered at 5 μM and 50 μM of cisplatin co-administered with the highest conventional creatine dose, 1 mmol/L i.e., decrease activity means more survival and higher cell viability (Figure 2A).
Kre-Alkalyn®, a buffered creatine led to a prominent, statistically significant and dose-dependent amelioration of cisplatin cytotoxicity in the 293T cells. In all treatment groups the combination of cisplatin and buffered creatine (Kre-Alkalyn®) at both concentrations, was associated with significantly higher cell viability as compared to the effects of cisplatin alone (Figure 2B).
The stability of a given compound under the chosen experimental conditions is a crucial prerequisite for optimal activity in vitro and in vivo. Historical data indicating the degradation of creatine to creatinine in acidic pHs [16] which was replicated here. Creatinine, which is excreted in the urine, is increased in the serum during kidney failure and is a main measure for assessment of renal function. Increases in serum creatine-creatinine are also seen after cardiac surgery, and in a variety of creatine synthesis and transport disorders [1,33,34], as well as inborn metabolic disorders of creatine synthesis and kidney function [34-36]. This breakdown has been associated with the pH of the microenvironment [1,37] . However, under more complex physiological conditions, and in healthy volunteers, the breakdown of creatine to creatinine seems to be well controlled regardless of pH [4,38]. This data and others suggest that the in vivo regulation of creatine synthesis and degradation is highly regulated in vivo. Yet only a small fraction of ingested creatine reaches the tissues [6].
The rate of transport into the cell by the predominant creatine transporter SLC6A8 is around 160 mmol/kg for each muscle cell, allowing degradation and other factors to play a role [3,7,39,40]. In the typically acidic small intestine, 99% of the esterified form of creatine will be degraded back to creatine and thus have no increased effect in reaching the muscle cells [4]. The ‘Holy Grail’ of creatine research therefore could depend on alkalizing the stomach or creating a micro-alkaline environment. This may aid in allowing the creatine to enter proximity to target tissues before intramolecular cyclization.
Alkalinity may have additional beneficial properties as well. Alkaline changes in pH have been reported to reduce the viability of microorganisms and decrease the growth of tumor cells [20,26]. Therefore, gastric tumors may be inhibited by alkalizing the stomach and, though extremophile bacterial like H. pylori frequent the gastric system [26,27], most bacterial growth may be reduced as well. Further, reduced bacterial contamination during storage may result from alkaline buffering as well. Alkalinity may contribute to prevention of creatine breakdown during a long shelf life or after dissolution in water in which lower pH leads to increased creatine solubility and degradation [2]. In addition the activity of alkaline buffered creatine does not inhibit the functionally relevant characteristics of creatine as shown by the increase in proteins synthesis seen in both traditional creatines and buffered creatine (Kre-Alkalyn®), in human muscle and cartilage cells (Figure 1B), a finding that is corroborated by the similar physiological benefits of both traditional and alkalized creatine [18].
Finally in this report an examination of the role of buffered creatine on cisplatin-mediated cellular cytotoxicity was conducted. Cisplatin has been well established as a cancer therapy, including gastric cancer, the second most common cause of cancer death in the world [41]. However, administration of cisplatin has been shown to significantly increase the creatinine released in the urine as a result of kidney damage [42]. The current study looked at the effect of creatine and buffered creatine (Kre-Alkalyn®), on the cytotoxic effect elicited on a well-studied kidney cell line. Creatine, both conventional and buffered is non cytotoxic toward human kidney cell line 293T in a wide range of concentrations (Figure 2).
Significant cytoprotective potential against cisplatin toxicity was noted as a result of alkaline buffering. While conventional creatine displayed only marginal activity, the stabilized buffered creatine formulation(Kre-Alkalyn®) proved to afford effective protection of 293T cells against the deteriorating effects of cisplatin, a finding which could be ascribed to the superior stability of the buffered formulation under the conditions of our experimental setup or due to the effect of buffering itself (Figure 2B).
The alkalinity of the reagent (Kre-Alkalyn®) may be useful for counteracting the cytotoxic effect of cisplatin on the kidney and other organs during tumor treatment [43]. Previous studies have shown the positive effects of alkaline buffering on reductions in tumor growth rate [20], for example, which in combination with reduced cisplatin toxicity may be advantageous in cancer therapy.
The observations for Kre-Alkalyn® supports the concept that an alkaline environment in and of itself has an impact on cell membrane, cell behavior, as well as molecular stability. Introducing a treatment substance, in an alkaline buffered environment, contributed toward a positive impact with the up regulation of protein synthesis, and increased survival after toxic exposure to an anti-neoplastic substance. A pH modified strategy warrants further investigation in a number of uses and should be considered when designing an anti-neoplastic therapy modality as well as increased absorbent efficacy. Based on these encouraging results, additional studies should be attempted to examine the tumor suppressive and cytoprotective effects of alkaline buffering. Further, creatine administered in an alkaline buffered solution may be advantageous for patients specifically undergoing cisplatin chemotherapy who have preexisting kidney damage.
- Golini J (2006) The Dangers of Creatinine, Brandenton, FL: Booklocker.com, Inc.
- Jäger R, Purpura M, Shao A, Inoue T, Kreider RB (2011) Analysis of the efficacy, safety, and regulatory status of novel forms of creatine. Amino Acids 40: 1369-1383. [Crossref]
- Brosnan JT, Brosnan ME (2010) Creatine metabolism and the urea cycle. Mol Genet Metab 100 Suppl 1: S49-52. [Crossref]
- Hageböck M, Stahl U, Bader J (2014) Stability of creatine derivatives during simulated digestion in an in vitro model. Food Funct 5: 359-363. [Crossref]
- Kepler EJ (1929) Creatine-creatinine metabolism: general review with a contribution to the study of creatinuria in hyperthyroidism, in Department of Medicine, University of Minnesota/Mayo Foundation 37.
- MacNeil L, Hill L, MacDonald D, Keefe L, Cormier JF, et al. (2005) Analysis of creatine, creatinine, creatine-d3 and creatinine-d3 in urine, plasma, and red blood cells by HPLC and GC-MS to follow the fate of ingested creatine-d3. J Chromatogr B Analyt Technol Biomed Life Sci 827: 210-215. [Crossref]
- Smith RN, Agharkar AS1, Gonzales EB2 (2014) A review of creatine supplementation in age-related diseases: more than a supplement for athletes. F1000Res 3: 222. [Crossref]
- Shao A, Hathcock JN (2006) Risk assessment for creatine monohydrate. Regul Toxicol Pharmacol 45: 242-251. [Crossref]
- Cooper R, Naclerio F, Allgrove J, Jimenez A (2012) Creatine supplementation with specific view to exercise/sports performance: an update. J Int Soc Sports Nutr 9: 33. [Crossref]
- Garbati P, Salis A, Adriano E, Galatini A, Damonte G, et al. (2013) A new method to synthesize creatine derivatives. Amino Acids 45: 821-833. [Crossref]
- Perasso L, Lunardi GL, Risso F, Pohvozcheva AV, Leko MV, et al. (2008) Protective effects of some creatine derivatives in brain tissue anoxia. Neurochem Res 33: 765-775. [Crossref]
- Moraes Rd, Van Bavel D, Moraes BS, Tibiriçá E1 (2014) Effects of dietary creatine supplementation on systemic microvascular density and reactivity in healthy young adults. Nutr J 13: 115. [Crossref]
- Antonio J, Ciccone V (2013) The effects of pre versus post workout supplementation of creatine monohydrate on body composition and strength. J Int Soc Sports Nutr 10: 36. [Crossref]
- Benzi G (2000) Is there a rationale for the use of creatine either as nutritional supplementation or drug administration in humans participating in a sport? Pharmacological Research 41: 255-264.
- Claudino JG, Mezêncio B, Amaral S, Zanetti V, Benatti F, et al. (2014) Creatine monohydrate supplementation on lower-limb muscle power in Brazilian elite soccer players. J Int Soc Sports Nutr 11: 32. [Crossref]
- Schaufelberger M, Swedberg K (1998) Is creatine supplementation helpful for patients with chronic heart failure? Eur Heart J 19: 533-534. [Crossref]
- Katseres NS, Reading DW, Shayya L, Dicesare JC, Purser GH (2009) Non-enzymatic hydrolysis of creatine ethyl ester. Biochem Biophys Res Commun 386: 363-367. [Crossref]
- Jagim AR, Oliver JM, Sanchez A, Galvan E, Fluckey J, et al. (2012) A buffered form of creatine does not promote greater changes in muscle creatine content, body composition, or training adaptations than creatine monohydrate. J Int Soc Sports Nutr 9: 43. [Crossref]
2021 Copyright OAT. All rights reserv
- Nomura A, Zhang M, Sakamoto T, Ishii Y, Morishima Y, et al. (2003) Anti-inflammatory activity of creatine supplementation in endothelial cells in vitro. Br J Pharmacol 139: 715-720. [Crossref]
- Golini J, Jones WL (2014) Buffered vs. Non-Buffered Aliphatic Fatty Acids and their Anti-Proliferative Effects in Human Tumor Cell Lines. Single Cell Biology 18.
- Golini J, Jones WL (2014) Kre-Celazine(®) as a viable treatment for juvenile rheumatoid arthritis/juvenile idiopathic arthritis - a pilot study. J Med Food 17: 1022-1026. [Crossref]
- [Estrella V, Chen T, Lloyd M, Wojtkowiak J, Cornnell HH, et al. (2013) Acidity generated by the tumor microenvironment drives local invasion. Cancer Res 73: 1524-1535. [Crossref]
- Vaupel P, Kallinowski F, Okunieff P (1989) Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res 49: 6449-6465. [Crossref]
- McCarty MF, Whitaker J (2010) Manipulating tumor acidification as a cancer treatment strategy. Altern Med Rev 15: 264-272. [Crossref]
- Silva AS, Yunes JA, Gillies RJ, Gatenby RA (2009) The potential role of systemic buffers in reducing intratumoral extracellular pH and acid-mediated invasion. Cancer Res 69: 2677-2684. [Crossref]
- Padan E, Bibi E, Ito M, Krulwich TA (2005) Alkaline pH homeostasis in bacteria: new insights. Biochim Biophys Acta 1717: 67-88. [Crossref]
- Saito H, Kobayashi H (2003) Bacterial responses to alkaline stress. Sci Prog 86: 271-282. [Crossref]
- Rossi F, Gaio E, Torriani S (2010) Staphylococcus aureus and Zygosaccharomyces bailii as primary microbial contaminants of a spoiled herbal food supplement and evaluation of their survival during shelf life. Food microbiol 27: 356-362. [Crossref]
- Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65: 55-63. [Crossref]
- Konstantinov SM, Eibl H, Berger MR (1999) BCR-ABL influences the antileukaemic efficacy of alkylphosphocholines. Br J Haematol 107: 365-380. [Crossref]
- Momekov G, Ferdinandov D, Bakalova A, Zaharieva M, Konstantinov S, et al. (2006) In vitro toxicological evaluation of a dinuclear platinum(II) complex with acetate ligands. Arch Toxicol 80: 555-560. [Crossref]
- Meijer S, Sleijfer DT, Mulder NH, Donker AJ (1982) Cisplatin-induced nephrotoxicity. Neth J Med 25: 262-269. [Crossref]
- Najafi M (2014) Serum creatinine role in predicting outcome after cardiac surgery beyond acute kidney injury. World J Cardiol 6: 1006-1021. [Crossref]
- Auray-Blais C, Maranda B, Lavoie P (2014) High-throughput tandem mass spectrometry multiplex analysis for newborn urinary screening of creatine synthesis and transport disorders, Triple H syndrome and OTC deficiency. Clin Chim Acta 436: 249-255. [Crossref]
- Wu HM, Sun HJ, Wang F, Yang M, Dong BR, et al. (2014) Oral adsorbents for preventing or delaying the progression of chronic kidney disease. Cochrane Database Syst Rev 10: CD007861. [Crossref]
- Ogedegbe HO (2007) Renal Function Tests: A Clinical Laboratory Perspective. Lab Medicine 38: 295-304.
- Niesser M, Koletzko B, Peissner W (2012) Determination of creatinine in human urine with flow injection tandem mass spectrometry. Ann Nutr Metab 61: 314-321. [Crossref]
- Ropero-Miller JD, Paget-Wilkes H, Doering PL, Goldberger BA (2000) Effect of oral creatine supplementation on random urine creatinine, pH, and specific gravity measurements. Clinical chemistry 46: 295-297. [Crossref]
- Russell AP, Ghobrial L, Wright CR, Lamon S, Brown EL, et al. (2014) Creatine transporter (SLC6A8) knockout mice display an increased capacity for in vitro creatine biosynthesis in skeletal muscle. Front Physiol 5: 314. [Crossref]
- Brault JJ, Terjung RL (2003) Creatine uptake and creatine transporter expression among rat skeletal muscle fiber types. Am J Physiol Cell Physiol 284: C1481-1489. [Crossref]
- Bilici A (2014) Treatment options in patients with metastatic gastric cancer: current status and future perspectives. World J Gastroenterol 20: 3905-3915. [Crossref]
- Ahn TG, Kim HK, Park SW, Kim SA, Lee BR, et al. (2014) Protective effects of green tea polyphenol against cisplatin-induced nephrotoxicity in rats. Obstet Gynecol Sci 57: 464-470. [Crossref]
- Oh GS, Kim HJ, Shen A1, Lee SB, Khadka D, et al. (2014) Cisplatin-induced Kidney Dysfunction and Perspectives on Improving Treatment Strategies. Electrolyte Blood Press 12: 55-65. [Crossref]


