Abstract
The Parkinson’s disease (PD) is a neurodegenerative disorder accompanied by movement-related, neuropsychiatric and vegetative problems. Progressive disease development, inadequate therapy, heavy disablement of the majority of patients turns PD into a serious social issue. The objective of the present work was the study of the oxidative destruction of brain proteins and lipids, аnd contents of neutral glycolipids in laboratory animals (rats) with experimentally induced syndrome of PD and under the treatment with lithium salt of cysteine. The experimental PD syndrome was induced by 1-methyl-4-phenyl-1,2,3,6-tetrapyridine. It was shown that the development of PD leaded to an increase in hydroperpxides and malone dialdehyde in brain. and protein carbonyl derivatives in plazma. The levels of aliphatic aldehyde and ketone dinitrophenyl hydrasones increased, that suggested the acceleration of oxidative destruction of proteins. Changes in neutral glycolipid metabolism result in a decreased expression of cerebrosides and sulfatides. Also, an increase in the sphingosine level (the product of hydrolysis of neutral glycolipids) was observed in brain. Disorders in lipid metabolism are thought to play an essential role in pathology of PD. The application of lithium salt of cysteine leads to a partial normalization of free radical oxidation and content of neutral glycolipids
Key words
Experimental PD syndrome, lipid peroxide, cerebrosides, sphingosine, lithium salt of cysteine
Introduction
It is well known that the aging process is accompanied by the development of age-related pathological processes, such as stroke, cerebrovascular accidents, atherosclerotic encephalopathy, malignant growth, endocrine and immune disorders. Such forms of age-related pathologies of the CNS as depression, Parkinson's disease, Alzheimer's disease and senile dementia are important today both in the medical and social terms. It is reasonably to note the substantial "rejuvenation" of diseases mentioned in recent years, the increase in the incidence of these diseases in 40-60-aged, that suggests the untimely early development of age-related changes of the brain.
Changes that occur in the brain during aging increase the risk of Parkinson’s disease. This disease is one of the most common neurodegenerative disorders; they are characterized by a selective degeneration of dopaminergic neurons in the substantia nigra. This disease is aprogressive neurodegenerative condition, which is characterized primarily by the four cardinal motor symptoms: resting tremor, bradykinesia, rigidity and postural instability. Non-motor features include cognitive impairment, hallucinations, autonomic dysfunction and sleep disorders. In neurodegenerative diseases generally and particularly in PD, abnormalites in mitochondrial functions have been linked to the pathological mechanism of these diseases [1,2].
Accelerated generation of reactive oxygen species (ROS) is one of conditions for age-related diseases and causes of oxidative stress, where formation of oxidants prevails the ability of antioxidant systems to remove ROS [3,4]. Many metabolic signaling pathways become dysfunctional because of increased oxidative stress and as consequence of that accumulation of oxidatively damaged molecules [5]. One of such oxidative damages at age-related conditions is oxidative modifications of proteins, which makes them dysfunctional. Accumulation of such kind proteins causes disruption of cellular functions. Protein oxidation can be induced directly by ROS or indirectly by reaction with secondary by-products of oxidative stress (generated from lipid peroxidation, sugars oxidation, etc.) [6,7].
Lipids are not only structural components of cell membranes, they also play an important role in their functional activity. The activity of membrane enzymes and receptors, as well as cell phagocytosis and adhesion, depend on the properties of the lipid membrane, such as viscosity, surface charge, and polarity. Impaired lipid metabolism in an organism can result in a number of age-related disease pathological processes in humans and animals.
The objective of this study was to examine the free radical-induced lipid oxidation, oxidative protein degradation and quantitative changes in neutral lipid (cerebrosides and sulphocerebrosides) contents in brain tissue at PD.
Lithium salts are well known remedy applied at neuro-degenerative conditions [8]. Li ions were demonstrated to increase the sensitivity of brain neurons to dopamine. Li competes with Na ions and participates in the regulation of mitochondrial Ca-channel activity; it also suppresses apoptotic cascades in the cells [9,10] present study the effect of a newly synthesized lithium salt of cysteine on the indices listed above was studied as well.
Material and methods
Old (2-2.5 years) white rats were used in the experiments. The animals were kept in accordance with the rules of the European Convention on the Protection of Vertebrates Used in Experimental and Research Purposes [11]. The model of the experimental syndrome of Parkinson's disease (SPD) was performed by every day intraperitoneal injection of 1-methyl-4-phenyl-1, 2, 3, 6-tetrapyridine (MPTP) (25.0 mg/kg).
Rats were separated into three groups. Each group consisted of 15 rats. The first group of intact animals was used as a control, second group was animals with experimentally SPD, and third group – animals with SPD treated with lithium salt of cysteine. This substance at the dose of 25 mg/kg was injected from the 6th to the 15th days of the experiment, every time 1 h after injection of MPTP. The animals were killed at 15th day of the experiment.
Lipid peroxidation (LPO) was measured in total brain homogenate (Tris - HCl –buffer) by the level of generated hydrogenperoxides (HP) and malondialdehyde (MDA). The level of HP was determined calorimetrically in reaction with ammonium thiocyanate detected at 480 nm [12]. MDA was detected by the reaction with thiobarbituric acid [12]. The protein content was determined according to the Lowry procedure [13].
The peroxidation processes were studied in enzymatic (NADPH-dependent) and non-enzymatic (ascorbic acid-dependent) oxidation systems. In the former reduced NADPH is used as a donor of reducing equivalents; in the latter ascorbic acid is used as a reducing agent.
Oxidative modification of proteins in plasma was estimated via measurement of absorption spectrum of 2,4-dinitrophenylhydrazine (DNPH) derivatives that were generated through interaction of DNPH with carbonyl derivatives of oxidized proteins (Levin’s method) [14]. For receiving real values of DNPH derivatives two measurements were done: in first one measured sample contain 0.1 ml plasma of blood, 1 ml DNPH and 0.9ml 20% trichloroacetic acid (TCA), in second one instead of 1ml DNTP there were 1ml 2N HCl. These samples were incubated during 1 hour by shaking in room temperature, and then were centrifuged. Precipitates were washed three times with ethanol:ethyl acetate (1:1) solution, then were dried and suspended in 9 mol solution of urea and 5min stayed in boiling water. The absorption was measured at 356 nm, 370 nm, 430 nm and 530 nm.
Lipid extraction in brain was performed according to Folch’s method.
The precipitation of cerebrosides and sulfatides depends on their ability to form a dense white layer at the interface of water and chloroform layers following the chloroform_methanol lipid extract treatment with trichloroacetic acid and water. Fractionation of cerebrosides and sulfatides was performed by TLC (TLC plates, Merck, Germany) using chloroform: methanol: concentrated ammonia (80 : 20 : 0,4) as a mobile phase. The amount of cerebrosides was determined via the interaction of sugar residue with resorcin, and amount of sulfatide was determined via the interaction of the sulfate group with azure [15]. To isolate sphingosine residues, the mixture of cerebrosides and sulfatides was subjected to acidic methanolysis by an H2SO4: methanol mixture (1 : 20) at 78–80°C for 6 h, followed by the extraction of sphingosine residue with diethyl ether. The amount of sphingosine was determined by intensity of color producing in reaction with methyl orange; absorbance measurement was performed at wavelength 415 nm [16] The obtained results were analyzed using Student’s t test and expressed as mean ± SEM.
Results and discussion
It was shown that induced PD resulted in the elevation of HP and MDA in brain tissue (Table 1). These metabolites are known to be generated in the process of oxidative destruction of polyunsaturated fatty acids (linoleic, linolenolic and arachidonic), an important component of phospholipids of biological membranes.
|
NADPH-depentent |
Ascorbic acid-dependent |
|
Control |
SPD |
SPD + Li cysteine |
Control |
SPD |
SPD + Li cysteine |
Hydropero-
xides |
058 ± 0.05 |
0,88 ± 0,049* |
0,45 ± 0,03** |
0,79 ± 0,06 |
1,3 ± 0,1** |
0,66 ± 0,04** |
malondialdehyde |
5,66 ±
0,5 |
9,73 ± 0,7** |
6,5 ± 0,5*- |
7,32 ± 0,3 |
12,87 ±
0, 32** |
8,3 ± 0.5*- |
The values are means ± SEM - * - р< 0,001; **р < 0,01 |
Table 1. The level of lipid peroxides in brain (nm/mg of protein) at experimental SPD and at SPD treated with lithium salt of cysteine.
It is known that fatty acid molecules are rich of double bonds; that is why they easily undergo free radical oxidation. The fatty acid oxidation results in formation of hydroperoxides (dienic conjugates), then they metabolize into secondary (MDA) and tertiary products. The level of peroxidation products is dependent on the cellular antioxidant content. It is known that at PD substantia nigra contains less glial cells synthesizing the natural antioxidant glutathion peroxidase, than other parts of the brain; the decrease of glutathion level was also described [17]. The low antioxidant content corresponds with high level of cytotoxic ROS inhibiting sulphydryl groups of enzymes and damaging NН2-groups of membranous proteins.
Analysis of oxidative damage of proteins at PD shown the statistically reliable increase of protein carbonyl derivatives (Figure 1), particularly, aliphatic aldehyde and ketone DNPH This suggests the intensive oxidative protein destruction leading to the formation of stable metabolites of amino acids. The rate of oxidative protein modification is known to be determined by the amino acid composition of proteins. The acceptor groups able to capture electrons interact with ROS and generate anion radicals are disulphide, sulphhydryl, carbonyl, carboxyl and NH-groups [18].
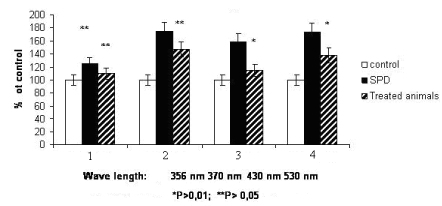
Figure 1. The amount of 2,4 –dinitrophenylhydrazine derivates in blood plasma at experimental SPD and SPD + Li cysteine.
- 1. neutral ketone derivatives of 2,4 - dinitrophenylhydrazine at 356 nm ( KDNPH )
- 2. neutral aldehyde derivatives of 2,4 - dinitrophenylhydrazine at 370 nm (ADNPH)
- 3. basic ketone derivatives of 2,4 - dinitrophenylhydrazine at 430 nm ( KDNPH )
- 4. basic aldehyde derivatives of 2,4 – dinitrophenylhydrazine 530 nm (ADNPH)
Oxidative damage of proteins plays an important role in ethiology of Parkinson’s and Alzheimer’s diseases. Few authors considered that the accumulation of oxidized damaged proteins take place at organism ageing; it is responsible for senile conditions (Parkinson’s and Alzheimer’s diseases, cataract, etc.). The mechanisms of wrong protein accumulation may be various. According to the literature [19] the generation of oxidized proteins is a typical result of effects of ROS which are normally produced by the cell metabolism. At PD the protein oxidative modification is accompanied by their aggregation and formation of inclusions resembling aggresomes (intracellular protein aggregates). These structures consist of polypeptide chains of proteins of irregular structure; they form insoluble nuclear and cytoplasmic bodies toxic for the cells.
It is known that the appearance of aggregated conglomerates may be evaluated by the level of basic and neutral KDNPH and protein fragmentation – by basic and neutral ADNPH [20]. Our results shown that PD development leads to increase of contents both KDNPH and ADNPH.
The development of oxidative processes brings to disorders of lipids components of cell membranes. To estimate the pattern of membranous lipids at PD the qualitative and quantitative spectrum of neutral glycolipids and the product of their hydrolytic dissociation sphingosine was studied. In the brain of control animals two factions of cerebrosides and two fractions of sulfatides were revealed; they differ in the content of fatty acids (Table 2). At PD a decrease in the content of both the total and fractional composition of neutral glycolipids was observed.
Table 2. The contents of glycosphingolipids and sphingosine in brain tissue at experimental SPD.
Glycosphingolipid classes |
Control |
SPD |
SPD + Li cysteine |
µg/g |
% of total |
µg/g |
% of total |
µg/g |
% of total |
Cerebroside sulfate- fraction 1 |
1.26 ± 0.09 |
7.66 |
0.7 ± 0.13** |
8,10 |
0,98 ± 0.07
|
7,10 |
Cerebroside sulfate- fraction 2 |
1.4 ± 0.12 |
8.52 |
0,88 ± 0.05* |
10,18 |
1,17 ± 0.08** |
8,47 |
Galactosylceramide- fraction 1 |
6.51 ± 0.4 |
39.62 |
3,37 ± 0.24* |
39,0 |
5,66 ± 0.23* |
40,98 |
Galactosylceramide- fraction 2 |
7.26 ± 0.38 |
44.18 |
3,69 ± 0.25* |
41,62 |
6,0 ± 0.3* |
43,44 |
Total glycosphingolipids |
16.43 |
|
8,64 |
|
13.81 |
|
Sphingosine |
3.23 ± 0.33 |
|
5,2 ± 0.24* |
|
3.7 ± 0.17* |
|
The values are means ± SEM (n = 12). * - р< 0,001; **р < 0,01
Cerebrosides are localized primarily in the myelin, whereas sulfatides are found in non-myelin white matter. The main function of myelin is the fast propagation of nerve impulses via axons surrounded by a myelin sheath. In addition to the transmission of nerve impulses, the myelin sheath serves as a source of nutrition for the nerve fibers and also provides structural support and protection for the nerve. The described decrease in the studied fractions at PD is believed to be one of the underlying causes of impaired brain function observed during this condition.
According to results obtained, a sharp drop (about two times) of the level of galactosylceramide and cerebroside sulfate fractions and simultaneous increment of sphingosine content took place in PD animals; this, most likely, resulted from high sphingomyelinase activity. Free sphingosine is formed from sphingomielin and cerebrosides via enzymatic cleavage by ceramidase and then sphingomyelinase forms sphingosine and fatty acid. Sphingomyelinase is found in almost all cell types, but the most of it localizes in the myelin of brain cells.
Recently published data suggest that the sphingomyelinase activation depends on oxidative processes in the cell. When free radical processes are activated, the sphingomyelinase activity increases, which leads to the accumulation of ceramide and sphingosine. Sphingosine participates in the regulation of cell proliferation and cell death because it can inhibit the activity of protein kinase C [21]. Ceramide and sphingosine are known to mediate apoptosis, and their accumulation in brain cells leads to the intensive cell death.
There are data shown that activity of sphingomyelinase depends on the level of antioxidative processes in cells and can be regulated by exogenous antioxidants [22]. In this study the cysteine and Li containing compound was applied as a treatment agent. Cysteine by itself participates in metabolism of glutathione which is a component of glutathione peroxidase and glutathione reductase. These enzymes play a crucial role in protecting cells from generation of highly reactive free radicals, particularly from lipid peroxidation products [17]. Our results demonstrated that the synthesized compound decreases the level of lipid peroxidation (Table 1); particularly, it is able to normalize the oxidative damage of proteins (Figure 1) and the glycolipids level (Table 2) in animals with PD.
It suggests that Li salt of cysteine could have a protective effect at PD. Due to its physiological nature this compound could be of pharmacological value because of its antioxidant effect and low toxicity. More detailed investigation should be done to reveal this compound influence at various pathological conditions characterized by disorders of oxidative processes.
Conclusion
The results obtained in this study on changes in lipid and protein oxidation and lipid metabolism can be useful for better understanding the mechanisms of PD. The newly synthesized Li salt of cysteine was demonstrated to diminish the intensity of oxidative processes at PD.
References
2021 Copyright OAT. All rights reserv
- Dauer W, Przedborski S (2003) Parkinson's disease: mechanisms and models. Neuron 39: 889-909. [Crossref]
- Jenner P, Olanow CW (2006) The pathogenesis of cell death in Parkinson's disease. Neurology 66: S24-36. [Crossref]
- Büeler H (2010) Mitochondrial dynamics, cell death and the pathogenesis of Parkinson's disease. Apoptosis 15: 101-108. [Crossref]
- Tsang AH, Chung KK (2009) Oxidative and nitrosative stress in Parkinson's disease. Biochim Biophys Acta 1792: 643-650. [Crossref]
- Giasson BI, Ischiropoulos H, Lee VM, Trojanowski JQ (2002) The relationship between oxidative/nitrative stress and pathological inclusions in Alzheimer's and Parkinson's diseases. Free Radic Biol Med 32: 1264-1275. [Crossref]
- Martínez A, Portero-Otin M, Pamplona R, Ferrer I (2010) Protein targets of oxidative damage in human neurodegenerative diseases with abnormal protein aggregates. Brain Pathol 20: 281-297. [Crossref]
- Davies MJ (2003) Singlet oxygen-mediated damage to proteins and its consequences. Biochem Biophys Res Commun 305: 761-770. [Crossref]
- Perez-Martinez DA (2009) The Role of Lithium in Neurodegenerative Diseases: New Registries for Old Actors. Neurologia 24: 143-146. [Crossref]
- Pérez M, Hernández F, Lim F, Díaz-Nido J, Avila J (2003) Chronic lithium treatment decreases mutant tau protein aggregation in a transgenic mouse model. J Alzheimers Dis 5: 301-308. [Crossref]
- Marmol F (2008) Lithium: bipolar disorder and neurodegenerative diseases Possible cellular mechanisms of the therapeutic effects of lithium. Prog Neuropsychopharmacol Biol Psychiatry 32: 1761-1771. [Crossref]
- European convention for the protection of vertebrate animals used for experimental and other scientific purposes (1986) Strasbourg 18 III, France.
- Orekhovich VN (1977) Modern Methods in Biochemistry (in russian). Medicina, Moscow. p. 62-66.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265-275. [Crossref]
- Levine RL, Garland D, Oliver CN, Amici A, Climent I, et al. (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186: 464-478. [Crossref]
- Keits M (1975) The Method of Lipidology. Mir, Moscow. p. 210.
- Prokhorova M (1982) The Methods of Biochemical Studies. Leningrad University. Leningrad. p. 271.
- Damier P, Hirsch EC, Zhang P, Agid Y, Javoy-Agid F (1993) Glutathione peroxidase, glial cells and Parkinson's disease. Neuroscience 52: 1-6. [Crossref]
- Rubinsztein DC (2006) The roles of intracellular protein-degradation pathways in neurodegeneration. Nature 443: 780-786. [Crossref]
- Danielson SR, Andersen JK (2008) Oxidative and nitrative protein modifications in Parkinson's disease. Free Radic Biol Med 44: 1787-1794. [Crossref]
- Chakravarti B, Chakravarti DN (2007) Oxidative modification of proteins: age-related changes. Gerontology 53: 128-139. [Crossref]
- Feng X, Becker KP, Stribling SD, Peters KG, Hannun YA (2000) Regulation of receptor-mediated protein kinase C membrane trafficking by autophosphorylation. J Biol Chem 275: 17024-17034. [Crossref]
- Tsyupko AN, Dudnik LB, Evstigneeva RP, Alessenko AV (2001) Effects of reduced and oxidized glutathione on sphingomyelinase activity and contents of sphingomyelin and lipid peroxidation products in murine liver. Biochemistry (Mosc) 66: 1028-1034. [Crossref]

