Our previous study demonstrated the prophylactic effects of liraglutide, a glucagon-like peptide-1 analog, on the development of hyperglycemia in Wistar Bonn Kobori diabetic fatty (WBN/Kob-Leprfa, WBKDF) rats with obesity and pancreatitis when the treatment is initiated from their pre-diabetic stage. In the present study, we investigated whether liraglutide shows these therapeutic effects on hyperglycemia in overtly diabetic WBKDF rats. Male 14-week-old WBKDF rats (n = 28) were divided into 4 groups that received either saline or liraglutide (75, 150, and 300 μg/kg) subcutaneously, once daily for 4 weeks. Age-matched male Wistar rats (n = 7) were used as the normal control. WBKDF rats exhibit markedly higher plasma glucose levels, food intake and body weight at 14 weeks of age compared with Wistar rats, and retained these metabolic phenotypes during the treatment with the vehicle for 4 weeks. The treatment with liraglutide (300 μg/kg) brought about partial reduction of hyperglycemia that was accompanied by decreased food intake and body weight. On the other hand, the liraglutide treatment improved neither glucose intolerance nor insulin secretion despite a slight hypertrophy of pancreatic β-cell-type islet cells observed after the liraglutide treatment for 4 weeks. Therefore, it is likely that liraglutide has therapeutic effects on hyperglycemia even in diabetic WBKDF rats via its inhibitory action on food intake.
liraglutide, diabetes, obesity, rat, insulin
GLP-1: Glucagon-Like Peptide-1; IVGTT: Intravenous Glucose Tolerance Test; T2DM: Type 2 Diabetes Mellitus; WBKDF: Wistar Bonn Kobori Diabetic Fatty
Type 2 diabetes mellitus (T2DM), which is characterized by insulin resistance and β-cell dysfunction, has become a major worldwide public health problem owing to changes in human behavior and lifestyle. In order to understand the molecular basis of several factors that potentiate T2DM and to identify the effectiveness of newly investigated therapeutic agents against T2DM, suitable animals models are used as indispensable tools to elucidate the pathogenesis and associated consequences of T2DM [1].
The Wistar Bonn Kobori (WBN/Kob) rat has been shown to develop hyperglycemia, hypoinsulinemia, and glucose intolerance after the age of 17 months [2,3]. However, this model does not exhibit signs of hyperlipidemia, which is typical of diabetes. On the other hand, the Zucker fatty rat develops obesity, insulin resistance, hyperlipidemia [4]. Therefore, a new strain WBN/Kob-Leprfa (WBKDF) was developed by introducing the fa allele of the leptin receptor from the Zucker fatty rat into the parental WBN/Kob rat genome [5]. Compared to the parental strains, WBKDF rats are characterized by an earlier onset of diabetes, more severe pancreatic complications, and endogenous insulin resistance [5-8].
Glucagon-like peptide-1 (GLP-1), one of the major incretin hormones, enhances insulin secretion and regulates glucose homeostasis by inhibiting glucagon secretion and controlling satiation, thereby ultimately controlling body weight [9,10]. Once-daily administration of liraglutide to T2DM patients with obesity led to a significant reduction in glucose levels and body weight [11-13]. Although prophylactic effects of liraglutide on the progression of T2DM were reported in T2DM rat models with obesity [8,14,15], little information is available on the therapeutic effects of this drug in overtly diabetic rats. We therefore investigated the effects of liraglutide in WBKDF rats at 14−18 weeks of age, the state at which the animals exhibit significant hyperglycemia and obesity.
Test animals and growth conditions
All animal experimental procedures were carried out in accordance with the principles of laboratory animal care and approved by the Ethics Committee of Azabu University (Kanagawa, Japan). Five-week-old male WBKDF and Wistar rats (SPF grade) were obtained from Japan SLC (Shizuoka, Japan). To measure food and water intake in individual animals, all rats were housed singly in standard plastic cages (38 × 23 × 18 cm) lined with wood chips (White flake; Charles River Japan) under the following conditions: temperature, 21 ± 2 °C; humidity, 55% ± 5%; and lights on for 12 h daily from 08:00 to 20:00. They were allowed free access to standard chow (CRF1; Charles River Laboratories Japan, Kanagawa, Japan) and tap water from a plastic water bottle.
Research protocol
Fourteen-week-old WBKDF rats (n = 28) were allocated into 4 groups: (1) a vehicle (0 μg/kg) group (n = 7), (2) a low-dose (75 μg/kg) liraglutide group (n = 7), (3) a medium-dose (150 μg/kg) liraglutide group (n = 7) and (4) a high-dose (300 μg/kg) liraglutide group (n = 7). Age-matched male Wistar rats (n = 7) were used as a normal control group. Vehicle or liraglutide (Victoza; Novo Nordisk Pharma, Tokyo, Japan) was administered subcutaneously to WBKDF rats once daily for 4 weeks. These protocols are based on the results of our previous studies [7,8].
Blood sampling and plasma separation
Blood samples were taken once weekly from the tail vein of the non-fasting and conscious rats at 6 and 14–18 weeks of age. At the end of the experiment, blood samples were drawn from the abdominal aorta under pentobarbital anesthesia. Heparin sodium (Mitsubishi Tanabe Pharma, Tokyo, Japan) was used as an anticoagulant, and plasma was separated from the collected blood by centrifugation at 3000 × g for 10 min.
Intravenous glucose tolerance test
The intravenous glucose tolerance test (IVGTT) was performed in all rats at the end of the study when the rats turned 18-weeks-old, as described previously [8]. Briefly, after fasting for 18 h, the rats were anesthetized using pentobarbital sodium (50 to 60 mg/kg IP). Glucose (20% w/v; Otsuka Pharmaceutical, Tokyo, Japan) was injected into the femoral vein of the rats, at a dose of 0.5 g/kg. Blood samples (0.2 mL) were then collected at time intervals of 0, 2, 5, 10, and 20 min after injection from the jugular vein. Heparinized plasma was separated by centrifugation (2000 × g for 15 min) to determine plasma glucose and insulin levels. The area under the curves (AUCs) of plasma glucose and insulin during IVGTT in each group was derived according to the trapezoidal rule [16] and the differences between groups were compared.
Measurement of blood biomarkers
Plasma glucose levels were measured using the Glucose CII-Test Wako kit (Wako Pure Chemical Industries, Osaka, Japan). Plasma insulin levels were quantitated by rat insulin enzyme-linked immunosorbent assay (ELISA) kit (Morinaga Institute of Biological Science, Kanagawa, Japan).
Measurements of body weight, food consumption, and water intake
The body weights of the rats, and their food and water intakes were measured once weekly at 6 and 14–18 weeks of age between 10:00 and 14:00.
Measurements of fat content
After IVGTT was performed, all rats were given additional pentobarbital sodium and euthanized by exsanguination. The epididymal and mesenteric fat were harvested and immediately weighed.
Histopathological examination of the pancreas
Pathological examination of the pancreas was done as described previously [8,17,18]. Briefly, pancreatic tissue isolated during necropsy was fixed in a 10% neutral buffered formalin solution, then paraffin-embedded and thin-sectioned (3−5 μm thick) for routine histopathological and immunohistochemical examination. Hematoxylin & eosin (H&E) staining was performed following standard protocols and morphological changes were observed under a light microscope. In addition, tissue sections were stained with antibodies against insulin (A0564; DAKO Japan Ltd., Kyoto, Japan). Anti-insulin immunoreactivity was determined using the avidin-biotin-peroxidase complex method (LSAB2 Kit, catalog no. K0609; DAKO Japan Ltd., Kyoto, Japan) and the peroxidase-labeled polymer method (Histofine Simple Stain Rat MAX-PRO [MULTI]; Nichirei Bioscience, Tokyo, Japan), as described previously [8]. Histopathological examination was performed in a blinded manner.
Statistical analysis
All data are represented as means ± standard error of the mean (SE). Statistical analyses were performed using GraphPad Prism version 6.0 (GraphPad Software Inc, San Diego, CA, USA) by one-way analysis of variance (ANOVA), followed by post-hoc Tukey tests. Differences with a P-value of < 0.05 were considered significant.
Non-fasting plasma levels of glucose and insulin
The plasma glucose levels in Wistar rats aged 6 and 14 weeks were compared (120 ± 3 mg/dL at 6 weeks of age, and 109 ± 2 mg/dL at 14 weeks of age, n = 7), and were found to have remained stable between 14 and 18 weeks of age (Figure 1A). On the other hand, the plasma glucose levels in the WBKDF rats increased 4-fold during 8 weeks from 6 weeks of age (119 ± 3 mg/dL at 6 weeks old and 488 ± 10 mg/dL at 14 weeks old; n = 28, P < 0.01). There was no significant difference in plasma glucose levels among the four groups of WBKDF rats just before the initiation of vehicle or liraglutide administration. After initiation, plasma glucose levels remained stable during the 4-week administration period in the group treated with the vehicle (487 ± 22 mg/dL at 14 weeks of age, 505 ± 13 mg/dL at 15 weeks of age, 460 ± 14 mg/dL at 16 weeks of age, 483 ± 22 mg/dL at 17 weeks of age, and 496 ± 18 mg/dL at 18 weeks of age, n = 7). While the treatment with liraglutide at 75 µg/kg and 150 µg/kg did not alter plasma glucose levels in WBKDF rats (Figure 1A), treatment at 300 µg/kg reduced plasma glucose levels significantly (P < 0.01) within the week after treatment initiation and the reduced levels were maintained (503 ± 23 mg/dL at 14 weeks of age, 368 ± 21 mg/dL at 15 weeks of age, 365 ± 26 mg/dL at 16 weeks of age, 399 ± 21 mg/dL at 17 weeks of age and 397 ± 16 mg/dL at 18 weeks of age, n = 7) and were significantly (P < 0.01 at 17 weeks of age and P < 0.01 at 15, 16 and 18 weeks of age ) lower than in the vehicle group of WBKDF rats.
The plasma insulin levels in Wistar rats were compared between 6 and 14 weeks of age (2.0 ± 0.6 ng/mL at 6 weeks of age and 1.2 ± 0.5 ng/mL at 14 weeks of age, n = 7) and were found to have remained stable between 14 and 18 weeks of age (Figure 1B). The WBKDF rats exhibited marked hyperinsulinemia (15.9 ± 0.6 ng/mL, n = 28) at 6 weeks of age, after which plasma insulin levels decreased with age (2.2 ± 0.3 ng/mL at 14 weeks of age; n = 28, P < 0.01 vs 6 weeks of age). There was no significant difference between the four groups of WBKDF rats and Wistar rats at 14 weeks of age (Figure 1B). Liraglutide treatment did not alter plasma insulin levels, although plasma insulin levels in WBKDF rats treated with high-dose liraglutide were significantly (P < 0.05) higher than those in Wistar rats at 15 and 17 weeks of age (Figure 1B).
Food and water intake
At 14 weeks of age, food and water intake in the WBKDF rats were twice and 8-times higher, respectively, than the Wistar rats (Figure 1C, 1D) confirming that excessive food and water intake are behavioral symptoms of diabetes. No intergroup difference in food or water intake was observed among the 14-week-old WBKDF rats. Liraglutide suppressed food and water intake in a dose-dependent manner within one week (Figure 1C, 1D). Significant differences were observed between the vehicle group and the low-, medium- and high-dose liraglutide groups (P < 0.05; Figure 1C, 1D).
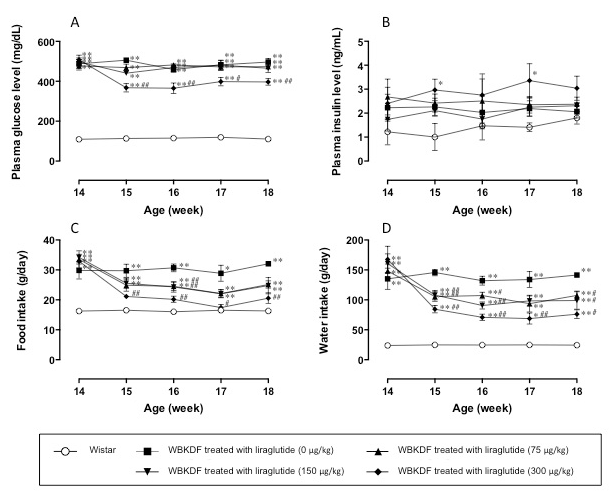
Figure 1. Effects of liraglutide on (A) plasma glucose concentrations, (B) plasma insulin concentrations, (C) daily food intake, and (D) daily water intake in male WBKDF rats 14 to 18 weeks of age. Data are expressed as mean ± SE (n = 7). *, P < 0.05; **, P < 0.01 versus Wistar rats. #, P < 0.05; ##, P < 0.01 versus WBKDF rats treated with liraglutide (0 μg/kg).
Intravenous glucose tolerance test
An IVGTT was performed on 18-week-old WBKDF and Wistar rats after 18-hr fasting. The plasma glucose levels of each of the four groups of WBKDF rats were significantly (P < 0.01) higher than those of Wistar rats, but no intergroup differences in plasma glucose levels among the four groups of WBKDF rats were observed at 0 min. Compared with the vehicle group of WBKDF rats, the low-dose liraglutide group exhibited significantly higher plasma glucose levels at 5 and 10 min after glucose loading (P < 0.01, Figure 2A).
The plasma insulin levels of WBKDF rats before and after glucose loading were significantly (P < 0.01, Figure 2B) lower than that in Wistar rats. No intergroup differences in plasma insulin levels among the four WBKDF rat groups were observed before and after glucose loading (Figure 2B).
The plasma glucose and insulin levels measured periodically during IVGTT were used to calculate AUCs as indexes of glucose intolerance and insulin secretion, respectively. The glucose AUC values of each of the four WBKDF groups were significantly higher than those of the Wistar group (P < 0.01; Figure 2C). The glucose AUC value of the low-dose liraglutide group was significantly higher than the vehicle group (P < 0.01; Figure 2C). The insulin AUC values of each of the four WBKDF rat groups were significantly lower than those of the Wistar group (P < 0.01; Figure 2D). There was no significant difference in insulin AUC value between the vehicle and liraglutide groups (Figure 2D).

Figure 2. Effects of liraglutide on plasma (A) glucose and (B) insulin concentrations and (C, D) their respective AUC values in male WBKDF rats undergoing IVGTT. Data are expressed as mean ± SE (n = 7). **, P < 0.01 P < 0.01 versus Wistar rats. ##, P < 0.01 versus WBKDF rats treated with liraglitide (0 μg/kg).
Body weight and fat content
At 14 weeks of age (just before the initiation of vehicle or drug administration), the body weights of WBKDF rats were significantly higher than that of the Wistar rats (P < 0.05: Figure 3A) and not significantly different among the four groups of WBKDF rats. After the initiation of treatment, while they linearly increased in the Wistar rats, the body weights only increased during the first 2 weeks and then remained unchanged during the rest of the treatment period in the vehicle group of WBKDF rats. At the end of the treatment period, there was no difference in body weight between the Wistar and the vehicle groups of WBKDF rats. In the low- and middle-dose liraglutide groups, the body weights did not increase and tended to be lower compared with those of the vehicle group of WBKDF rats over the treatment period. In the high-dose liraglutide group, the body weights significantly decreased by approximately 8% (364 ± 8 g at 14 weeks of age and 335 ± 10 g at 15 weeks of age, n=7, P<0.01) during the first week of treatment and remained significantly low compared with those of the vehicle group of WBKDF rats over the treatment period. The weights of the epididymis and mesentery fat tissues tended to decrease along with body weight with the liraglutide treatment (Figure 3B, 3C).
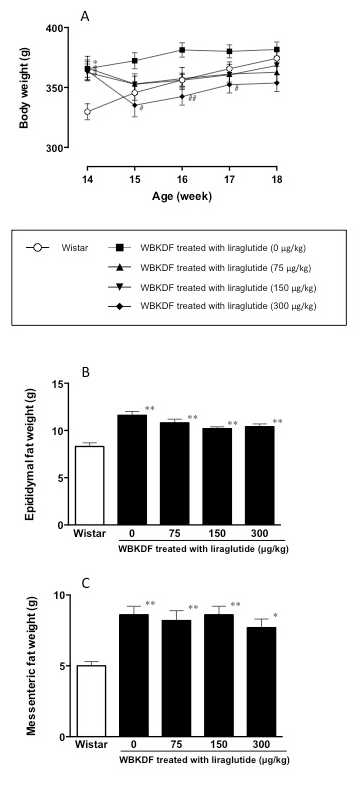
Figure 3. (A) Changes in body weight in male WBKDF rats treated with the vehicle or liraglutide from 14 to 18 weeks of age. Comparison of (B) epididymal and (C) mesenteric fat weight in WBKDF rats at 18 weeks of age. Data are expressed as mean ± SE (n = 7). *, P < 0.05; **, P < 0.01 versus Wistar rats. #, P < 0.05; ##, P < 0.01 versus WBKDF rats treated with liraglutide (0 μg/kg).
Histopathology of the pancreas
In the pancreas of the rats in the vehicle control and liraglutide groups, we noted evidence of mild-to-severe chronic interstitial pancreatitis, including interstitial fibrosis, proliferation of ductules, acinar cell atrophy, hemosiderosis, and lymphocytic infiltration (Figure 4A, 4C, 4E). Some large cells with weakly acidophilic cytoplasm were found scattered in the damaged areas and these cells stained positively for anti-insulin antibodies, indicating that interstitial pancreatitis spread to the islets. Although no clear effect of liraglutide on chronic interstitial pancreatitis was observed, the remaining pancreatic islets in the high dose group appeared relatively large. Immunohistochemical study showed that these enlarged islets were composed mainly of insulin-containing hypertrophic cells (Figure 4B, 4D, 4F).
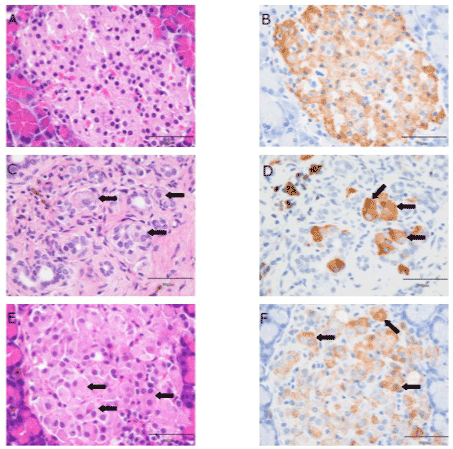
Figure 4. Histopathology and immunohistochemistry of the pancreas in normal Wistar rats (A and B) and WBKDF rats treated with (C and D) vehicle or (E and F) 300 µg/kg liraglutide at 18 weeks of age. (A, C, and E) H&E staining. (B, D, and E) Immunostaining for insulin. The pancreatic islets observed in the normal control Wistar rats appeared intact (A and B). In the WBKDF rats treated with the vehicle, chronic interstitial pancreatitis was observed, and large cells with weakly acidophilic cytoplasm (C, arrows) were positive for insulin staining (D, arrows). The remaining, enlarged islets observed in the 300 μg/kg/day group were composed of mainly hypertrophic cells (E, arrows), which were positive for insulin (F, arrows).
Liraglutide has been clinically used as a pharmacotherapy to improve glycemic control in T2DM patients, and its high-dose formulation has been approved for the treatment of chronic weight management [13]. Prophylactic effects of liraglutide on the progression of T2DM were reported in T2DM rat models with obesity, including Zucker diabetic fatty rats [14], Otsuka Long-Evans Tokushima fatty rats [15], and WBKDF rats [8], but little is known on the therapeutic effects of this drug in these rats. The current study demonstrated that liraglutide could reduce hyperglycemia partially even in overtly diabetic WBKDF rats at 14−18 weeks of age, which is characterized by severe hyperglycemia and insulin deficiency due to β-cell collapse.
The reduction of hyperglycemia by liraglutide was not accompanied by alteration of plasma insulin levels in the current study. This therapeutic effect of liraglutide is in sharp contrast to the preventive effects of this medication that we reported previously in pre-diabetic WBKDF rats [8]. The absence of an insulinotropic action of liraglutide in the present study seemed to be due to the advanced pancreatitis leading to severe dysfunction of islet β-cells in overtly diabetic WBKDF rats. WBKDF rats exhibit normal fasting glycemia with hyperinsulinemia as a compensatory response to insulin resistance in their pre-diabetic stage (~7 weeks of age) and with advancing age, hyperglycemia develops progressively with declining plasma insulin levels. The daily administration of liraglutide (37.5–150 μg/kg) from 7 weeks of age prevents the occurrence of hyperglycemia while maintaining hyperinsulinemia [6]. It has been reported that GLP-1 and its derivative expand pancreatic β-cell mass [14,19]. However, a large number of pancreatic β-cell type islet cells and epithelial cells of the small ducts in WBKDF rats treated with liraglutide were evident in the previous study [8], which is consistent with earlier studies reporting that GLP-1 and its derivative expand pancreatic β-cell mass [14,19]. Collectively, these results suggest that liraglutide treatment in pre-diabetic WBKDF rats prevents the occurrence of hyperglycemia while maintaining hyperinsulinemia by preserving the function of pancreatic β-cells (prophylactic effect), but fails to induce insulin-dependent anti-hyperglycemic effects in diabetic WBKDF rats with advanced pancreatic dysfunction. Thus, the therapeutic anti-hyperglycemic effects of liraglutide in diabetic WBKDF rats are unlikely due to its insulinotropic action.
GLP-1 receptor agonists are reported to have multiple functions, including enhancement of insulin secretion, and suppression of appetite, body weight, and gastric emptying [9,20]. Recently, liraglutide has been shown to enhance insulin sensitivity in rats [21]. There are several possible alternative mechanisms that can explain the therapeutic anti-hyperglycemic effects of high-dose liraglutide in diabetic WBKDF rats, i.e., insulin-independent improvement in glucose tolerance, improvement in insulin resistance, and suppression of hyperphagia. The IVGTT performed in fasted WBKDF rats revealed that liraglutide treatment produced no obvious improvement in glucose and insulin homeostasis. Thus, the anti-hyperglycemic effects of liraglutide were unlikely due to insulin-independent improvement in glucose homeostasis or insulin resistance.
It is well known that the oral administration of glucose yields higher GLP-1 release from the small intestine [9,22]. In the present study, to minimize the influences of intrinsic GLP-1 on GTTs, we performed IVGTT, but not oral GTT, on all rats under pentbarbithal sodium-induced anesthesia. However, previous studies reported that several anesthetics, including pentbarbithal sodium, may affect blood levels of glucose and insulin [23,24]. It was also reported that influences of anesthetics on blood levels of glucose and insulin may vary between fed and fasted rats [25]. It is suggested that anesthetic effects on blood blood glucose and insulin may be attenuated in the fasted condition [26]. Indeed, previous reports described that pentbaribithal sodium produced a transient increase in blood glucose in fed rats [24,27], but not in fasted rats [25,28,29]. Judging from these studies, it is possible, but less likely that pentbaribithal sodium significantly influenced plasma glucose and insulin levels in fasted WKBDF rats during the IVGTT.
The WBKDF rat carries the fatty mutation (fa) in the leptin receptor gene, and homozygous animals (fa/fa) exhibit hyperphagia and obesity in addition to insulin resistance and glucose intolerance [30-32]. There is substantial evidence that systemic administration of GLP-1 receptor agonists, including liraglutide, reduces food intake and body weight in experimental animals and humans with obesity [33,34]. The mechanism by which GLP-1 receptor agonists reduce food intake is suggested to be the suppression of appetite via GLP-1 receptors in the central nervous system, as well as in the intestinal tract [35,36]. The food intake of WBKDF rats was two-times higher than that of Wistar rats in the current study. The present study demonstrated that liraglutide treatment caused significant and dose-dependent reduction in food intake and body weight in diabetic WBKDF rats. It is noteworthy that plasma glucose levels in fasted WBKDF rats were comparable between high-dose liraglutide and vehicle groups. Therefore, these results suggest that the therapeutic anti-hyperglycemic effects of high-dose liraglutide in diabetic WBKDF rats are mainly due to the reduction in food intake.
In the current study performed using an animal model of obesity-associated T2DM, we demonstrated that liraglutide can reduce hyperglycemia even in the late phase of T2DM, which is characterized by severe hyperglycemia and insulin deficiency due to β-cell collapse, in WBKDF rats. It is suggested that liraglutide may have therapeutic effects by reducing hyperglycemia due to its inhibitory action on food intake rather than its insulinotropic action in WKDF rats.
FA is the principal investigator that designed and described all aspects of the study. DN, MS, KW, YN, NK all contributed to the manuscript by conducting experiments, analyzing data, and creating figures. DN and MS assisted in preparing the manuscript for submission, and contributed equally to this work.
2021 Copyright OAT. All rights reserv
The authors wish to thank Dr. Masakazu Shiota at Vanderbilt University for the insightful discussions.
This study was supported in part by a research project grant awarded by Azabu University.
The authors have no conflicts of interests to disclose.
- Akash MS, Rehman K, Chen S (2013) Goto-Kakizaki rats: its suitability as non-obese diabetic animal model for spontaneous type 2 diabetes mellitus. Curr Diabetes Rev 9: 387-396. [Crossref]
- Ohashi K, Kim JH, Hara H, Aso R, Akimoto T, et al. (1990) WBN/Kob rats. A new spontaneously occurring model of chronic pancreatitis. Int J Pancreatol 6: 231-247. [Crossref]
- Tago Y, Katsuta O, Tsuchitani M, Narama I, Itakura C (1991) Glomerular lesions in spontaneously occurring diabetic WBN/Kob rats. J Comp Pathol 104: 367-377. [Crossref]
- Zucker LM (1965) Hereditary obesity in the rat associated with hyperlipemia. Ann N Y Acad Sci 131: 447-458. [Crossref]
- Akimoto T, Nakama K, Katsuta Y, Zhang XJ, Ohsuga M, et al. (2008) Characterization of a novel congenic strain of diabetic fatty (WBN/Kob-Lepr(fa)) rat. Biochem Biophys Res Commun 366: 556-562. [Crossref]
- Kaji N, Okuno A, Ohno-Ichiki K, Oki H, Ishizawa H, et al. (2012) Plasma profiles of glucose, insulin and lipids in the male WBN/Kob-Lepr(fa) rat, a new model of type 2 diabetes with obesity. J Vet Med Sci 74: 1185-1189. [Crossref]
- Okuno A, Kaji N, Takahashi A, Nagakubo D, Ohno-Ichiki K, et al. (2013) Role of insulin resistance in the pathogenesis and development of type 2 diabetes in WBN/Kob-Lepr(fa) rats. J Vet Med Sci 75: 1557-1561. [Crossref]
- Nagakubo D, Shirai M, Nakamura Y, Kaji N, Arisato C, et al. (2014) Prophylactic effects of the glucagon-like Peptide-1 analog liraglutide on hyperglycemia in a rat model of type 2 diabetes mellitus associated with chronic pancreatitis and obesity. Comp Med 64: 121-127. [Crossref]
- Drucker DJ1 (2006) The biology of incretin hormones. Cell Metab 3: 153-165. [Crossref]
- Wajchenberg BL1 (2007) beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev 28: 187-218. [Crossref]
- Madsbad S, Schmitz O, Ranstam J, Jakobsen G, Matthews DR, et al. (2004) Improved glycemic control with no weight increase in patients with type 2 diabetes after once-daily treatment with the long-acting glucagon-like peptide 1 analog liraglutide (NN2211): a 12-week, double-blind, randomized, controlled trial. Diabetes Care 27: 1335-1342. [Crossref]
- Sisson EM (2011) Liraglutide: clinical pharmacology and considerations for therapy. Pharmacotherapy 31: 896-911. [Crossref]
- Ladenheim EE (2015) Liraglutide and obesity: a review of the data so far. Drug Des Devel Ther 9: 1867-1875. [Crossref]
- Sturis J, Gotfredsen CF, Rømer J, Rolin B, Ribel U, et al. (2003) GLP-1 derivative liraglutide in rats with beta-cell deficiencies: influence of metabolic state on beta-cell mass dynamics. Br J Pharmacol 140: 123-132. [Crossref]
- Guo N, Sun J, Chen H, Zhang H, Zhang Z, et al. (2013) Liraglutide prevents diabetes progression in prediabetic OLETF rats. Endocr J 60: 15-28. [Crossref]
- Wolever TM, Jenkins DJ (1986) The use of the glycemic index in predicting the blood glucose response to mixed meals. Am J Clin Nutr 43: 167-172. [Crossref]
- Taira K, Yazawa R, Watanabe A, Ishikawa Y, Okamoto M, et al. (2015) Syphacia muris infection delays the onset of hyperglycemia in WBN/Kob-Leprfa rats, a new type 2 diabetes model. Helminthologia 52: 58-62.
- Ogawa T, Sugidachi A, Tanaka N, Fujimoto K, Fukushige J, et al. (2005) Effects of R-102444 and its active metabolite R-96544, selective 5-HT2A receptor antagonists, on experimental acute and chronic pancreatitis: Additional evidence for possible involvement of 5-HT2A receptors in the development of experimental pancreatitis. Eur J Pharmacol 521: 156-163. [Crossref]
- Wang Y, Perfetti R, Greig NH, Holloway HW, DeOre KA, et al. (1997) Glucagon-like peptide-1 can reverse the age-related decline in glucose tolerance in rats. J Clin Invest 99: 2883-2889. [Crossref]
- Nauck MA, Niedereichholz U, Ettler R, Holst JJ, Orskov C, et al. (1997) Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol 273: E981-988. [Crossref]
- Yamazaki S, Satoh H, Watanabe T (2014) Liraglutide enhances insulin sensitivity by activating AMP-activated protein kinase in male Wistar rats. Endocrinology 155: 3288-3301. [Crossref]
- Schirra J, Katschinski M, Weidmann C, Schäfer T, Wank U, et al. (1996) Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Invest 97: 92-103. [Crossref]
- Sato K, Kitamura T, Kawamura G, Mori Y, Sato R, et al. (2013) Glucose use in fasted rats under sevoflurane anesthesia and propofol anesthesia. Anesth Analg 117: 627-633. [Crossref]
- Pénicaud L, Ferré P, Kande J, Leturque A, Issad T, et al. (1987) Effect of anesthesia on glucose production and utilization in rats. Am J Physiol 252: E365-369. [Crossref]
- Saha JK, Xia J, Grondin JM, Engle SK, Jakubowski JA (2005) Acute hyperglycemia induced by ketamine/xylazine anesthesia in rats: mechanisms and implications for preclinical models. Exp Biol Med (Maywood) 230: 777-784. [Crossref]
- Zuurbier CJ, Keijzers PJ, Koeman A, Van Wezel HB, Hollmann MW (2008) Anesthesia's effects on plasma glucose and insulin and cardiac hexokinase at similar hemodynamics and without major surgical stress in fed rats. Anesth Analg 106: 135-142, table of contents. [Crossref]
- Clark PW, Jenkins AB, Kraegen EW (1990) Pentobarbital reduces basal liver glucose output and its insulin suppression in rats. Am J Physiol 258: E701-707. [Crossref]
- Bailey CJ, Atkins TW, Matty AJ (1975) Blood glucose and plasma insulin levels during prolonged pentobarbitone anaesthesia in the rat. Endocrinol Exp 9: 177-185. [Crossref]
- Johansen O, Vaaler S, Jorde R, Reikerås O (1994) Increased plasma glucose levels after Hypnorm anaesthesia, but not after Pentobarbital anaesthesia in rats. Lab Anim 28: 244-248. [Crossref]
- Chua SC Jr, Chung WK, Wu-Peng XS, Zhang Y, Liu SM, et al. (1996) Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science 271: 994-996. [Crossref]
- Leonard BL, Watson RN, Loomes KM, Phillips AR, Cooper GJ (2005) Insulin resistance in the Zucker diabetic fatty rat: a metabolic characterisation of obese and lean phenotypes. Acta Diabetol 42: 162-170. [Crossref]
- Shiota M, Printz RL (2012) Diabetes in Zucker diabetic fatty rat. Methods Mol Biol 933: 103-123. [Crossref]
- Hermansen K, Bækdal TA, Düring M, Pietraszek A, Mortensen LS, et al. (2013) Liraglutide suppresses postprandial triglyceride and apolipoprotein B48 elevations after a fat-rich meal in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, cross-over trial. Diabetes Obes Metab 15: 1040-1048. [Crossref]
- van Bloemendaal L, Ten Kulve JS, la Fleur SE, Ijzerman RG, Diamant M (2014) Effects of glucagon-like peptide 1 on appetite and body weight: focus on the CNS. J Endocrinol 221: T1-16. [Crossref]
- Holst JJ (2007) The physiology of glucagon-like peptide 1. Physiol Rev 87: 1409-1439. [Crossref]
- Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR (2011) Peripheral and central GLP1 receptor populations mediate the anorectic effects of peripherally administered GLP1 receptor agonists, liraglutide and exendin-4. Endocrinology 152: 3103-3112. [Crossref]




