clinical trials, therapeutic exploratory trials, parallel group design
Clinical trials begin with phase 1 or first in human (FIH) trials which involve the evaluation of the pharmacokinetic and pharmacodynamic effects of a new drug in healthy volunteers. In addition, dose escalation studies are performed to evaluate the maximum tolerated dose in man which forms the basis of the dose(s) or dose ranges for use in phase 2 clinical trials [1]. Following phase 1 trials is the phase 2 trials also known as the therapeutic exploratory trials. This phase is conducted in about 100-200 patients with the disease condition under study using a narrow inclusion criteria [2,3]. According to Glasser therapeutic exploratory trials are feasibility studies which evaluate the efficacy, dose-response rate and response duration of the new drug, coupled with safety monitoring studies [4].
Some school of thought may consider the therapeutic exploratory trials as two phases, namely; phase 2a and phase 2b. Glasser [4] describes phase 2a as an extension of phase 1 trials in patients instead of healthy volunteers and phase 2b trials as small scale randomised controlled trials. Fitzpatrick and Downes, further explain that, phase 2a often employ dose escalation designs for the estimation of the dose response [5]. However, most often but not always the case, doses used in phase 2a studies are less than the highest dose employed in phase 1 studies. These may consist of concurrent control group, historical control group or baseline status comparisons [5]. Phase 2a trials also known as “proof-of-concept” also concentrate on proving the hypothesised mechanism of action of the new drug in fewer patients [6]. Progression unto phase 2b focuses on the use of parallel dose response designs for ‘dose-ranging’ studies to obtain the optimum tolerated dose (s) [5,6]. The therapeutic exploratory trials finally progress to phase 3 trials which are designed with a more formal hypothesis to confirm the efficacy and safety findings from the former trials [5].
In this write up, we consider the design of therapeutic exploratory trials with particular focus on parallel group designs and their usefulness in phase 2 of drug development.
A parallel group design is an experimental study design in which each subject is randomized to one of two or more distinct treatment/intervention groups [7]. Those who are assigned to the same treatment are referred to as a treatment group. The method of randomisation results in a number of variants of the parallel group design. Such variants include simple randomisation, blocked randomisation and stratified randomisation designs [3]. Other variants are cross-over and matched-pairs designs [5]. According to Chow and Lui [8], the choice of design depends on the suitability in addressing the investigational question. Nonetheless, the basic principle of experimental design; the principle of randomisation, is common in all parallel group designs. The International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) recommends the use of parallel group designs in the latter stages of the therapeutic exploratory trials or deferred to phase 3 trials [9]. On the contrary, Estey and Thall [10] in their paper; ‘New designs for phase 2 clinical trials’, contend the use of solely conventional single arm design and strongly recommends parallel groups designs in phase 2 trials on the premise of its failure to yield substantial reliable comparative results and a threat to the success of phase 3 trials.
Parallel group designs are therefore useful in therapeutic exploratory trials to fulfil the primary objective of evaluating the dose (s), dose regimen and safety of the investigational drug. Firstly, parallel group designs allow multi-arm comparisons, for example, a two-group or a three-group parallel design [8]. In these designs, efficacy of the treatment group is easily compared with multiple arms of the control group which may consist of different active controls and/or placebo control (Figure 1). This aids in the early identification of ineffective drugs or studies before progression into more extensive studies (phase 3) which require significant amount of cost, time and effort [11].
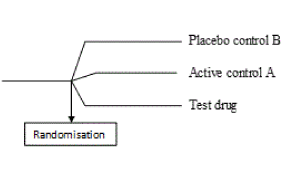
Figure 1. A three group parallel design with test treatment and control active control A and placebo control [8]
On the other hand, the multi-arm of parallel group design allows different fixed doses of the investigational drug to be compared to a placebo. This is significant in the determination of dose ranges in phase 2 trials based on the assessment of dose response [12]. Moreover, objectives of therapeutic exploratory trials may further consist of the evaluation of a subset of data such as concomitant regimen or target populations with varying degree of the disease condition [11]. For example, a subset of target population can be considered for the severity of the disease (mild, moderate and severe conditions) and allocated to different arms of the parallel group design with varying dose or dose ranges to evaluate its effectiveness in the intended target product profile. Again for disease conditions with delayed, persistent or irreversible endpoints such as stroke, heart attack prevention and arthritis, treatment, titration and simultaneous assessment of results is usually impossible. Hence, parallel group designs are more suited for such conditions because adequate time is allowed for dose-response comparisons [11]. Also, parallel group designs allow randomisation methods to be employed in the allocation of participants to test or control groups. In addition, double blinding is also easily achieved.
The above all add to minimise the occurrence of bias and statistically guarantees the reliability of the trial results which is key to speeding the drug development process [3]. Moreover, ethical issues with the use of placebo controls when an effective treatment is available can be overcome by employing an active control through the use of parallel drug design. Also, grouping of patients as opposed to individual subject in single-arm designs results in group mean responses [11]. This gives a better representation of a sample population to a study population (target of therapeutic exploratory trials), which usually consists of varied baseline characteristics. Parallel group designs are also universally accepted due to their simplicity, easy implementation and less complicated analysis and interpretation of results [10]. This is very key for easy transition into phase 3 trials which is usually focused on multicentre, multinational studies for globalisation and generalisation of study results.
In conclusion, parallel group design is useful in therapeutic exploratory trials because the design allows both the primary objectives; efficacy and safety studies, and further exploratory objectives consisting of subgroup analyses to be assessed more efficiently.
- Chin RY, Lee BY (2008) Principles and Practice of Clinical Trial Medicine. Oxford: Elsevier.
- Pocock JS (1995) Clinical trials: A practical approach. Wiley.
- Friedman LM, Furberg CD, DeMets DL (2010) Fundamentals of Clinical Trials. (4th Edn) Springer, New York.
- Glasser SP (2008) Essentials of Clinical Research. Springer, New York.
- Fitzpatrick S and Downes N (2015) Clinical Trial Design, Buckinghamshire: Institute of clinical research.
- Phase II (2015) Phase II Clinical Trials. Actelion.
- Parallel Group Study (2015) National institute for health research, Research design service south central.
- Chow S, Lui J (2008) Design and Analysis of Clinical Trials: Concepts and Methodologies. (2nd Edn) John Wiley & Sons, New Jersey.
- ICH (2015) International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. Topic E8: General considerations for clinical trials.
- Estey EH, Thall PF (2003) New designs for phase 2 clinical trials. Blood 102: 442-448. [Crossref]
- ICH (2015) International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Dose-Response Information to Support Drug Registration E4.
2021 Copyright OAT. All rights reserv
- ICH (2016) International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). Choice of Control Group and Related Issues in Clinical Trials E10.

