The case of a newborn with type V Aplasia Cutis Congenita is presented. The total body surface area affected by skin defects was about 18%. Dermal regeneration template was used to cover lesions, followed by keratinocytes graft. Patient outcome is presented after a follow-up of more than 2 years
aplasia cutis congenita, skin graft, IDRT
Aplasia Cutis Congenita (ACC) is a rare disorder affecting skin morphogenesis which results in skin defects at birth with incidence rate between 0.5 and 1/10’000 births. At least 500 cases are reported in the literature [1]. Different presentations of the defects exist and scalp defects are the most frequent. One form of ACC associated with either placental infarct or foetus papyraceous due to in utero death of a twin fetus, presents as extensive truncal and limb skin defects. This form was classified as Group V ACC according to Frieden [2] whose classification is based on the distribution of the affected area, type of inheritance and associated anomalies (Table 1). Class V is even rarer and only 45 cases are published in the western literature [1]. Lesions associated with Class V ACC are often bilateral and symmetrical, localized at the trunk, buttocks and thighs, in “H” or “stellate” configuration [2-5]. The pathophysiology of this disorder is poorly understood with at least five different theories on the etiology of Group V ACC. The most popular one is the feto-fetal transfusion [6]. A passage of blood from the surviving twin to the dying one due to his hypotension could cause a relative hypotension and hypovolemia in the living twin which would lead to ischemia of skin and other organs. Another suggestive theory is that the in utero death of one twin could cause the release of thrombosis-promoting material, thus causing placental infarcts, intravascular coagulation or fetal injuries [7].
Table 1. Frieden’s classification of aplasia cutis congenita (ACC) (extracted from {Frieden, 1986 569 /id})
|
Diagnosis |
1 |
Scalp ACC without multiple anomalies |
2 |
Scalp ACC with associated limb anomalies (limb reduction anomalies, 2-3 syndactyly, club-foot, nail absence or dystrophy, skin tags on toes, persistent cutis marmorata, encephalocele etc.) |
3 |
Scalp ACC with associated epidermal and organoid nevi (corneal opacitu, scleral dermoids, eyelid colobomas, psychomotor retardation, seizures) |
4 |
ACC overlying embryologic malformations (meningomyeloceles, spinal dysraphism, cranial stenosis, congenital midline, porencephaly, leptomeningealangiomatosis, ectopia of ear, omphalocele, gastroschisis) |
5 |
ACC with associated foetusparyraceus or placental infacts (these cases showed extensive truncal and limb involvement, usually symmetric and frequently with linear and stellate areas of aplasia) |
6 |
ACC associated with epidermolysisbullosa |
7 |
ACC localized to extremities without blistering |
8 |
ACC caused by specific teratogens (methymazole or carbimazole) |
9 |
ACC associated with malformation syndromes (trisomy 13, 4p- syndrome) |
We report here a case of Group V ACC and detail the management and outcome of the patient after more than 2 years.
A male newborn was admitted to the neonatal intensive care unit for ACC. The disease was associated with foetus papyraceus consecutive to intrauterine death of a monozygotic twin at the 12th week of pregnancy. The newborn weighed 2.985 g at birth and presented wide areas of skin defects in the back, abdomen, flanks, buttocks, trochanteric area and lower limbs. The total body surface area affected by the defects was about 18% (Figure 1). The patient was placed in an incubator and cared for in sterile conditions. He received parenteral nutrition and prophylactic antibiotherapy (amoxicillin and clavulanic acid). Nine days after birth, debridment of the affected areas followed by coverage using a skin substitute (Biobrane, Smith and Nephew, London, UK) was performed. Biopsies were taken from some areas of the defects which seemed to be covered with an epithelium-like membrane. Biopsy analysis showed stromal connective tissue with islands of epithelial cells with no true epithelium layer (Figure 2). Complete absence of epidermis, dermis and dermal appendages was also reported previously [8]. Fourteen days after birth, affected areas were debrided and grafted with double layer Integra Dermal Regeneration Template (IDRT, Integra LifeSciences, Plainsboro, NJ, USA) over flanks and abdomen (Figure 3). Also, a healthy skin biopsy was harvested to start a keratinocyte culture for further application. The IDRT graft took completely (Figure 4). At day 34 after the birth (20 days after IDRT application), the silicone layer was removed and autologous keratinocytes were applied on the grafted areas. The keratinocyte layer take was about 50%, because of a Staphilococcus aureus infection localized on the right side of the thorax. Infection was treated with Vancomycin and a new keraticocytes graft was performed 51 days after the birth (17 days after the first graft). This second keratinocyte application took only partially (about 50% again) and was followed by a split thickness skin graft (0.4 mm) from the buttock 12 days later. This last skin graft healed uneventfully (Figure 5). The baby left the hospital finally 3 months after birth.
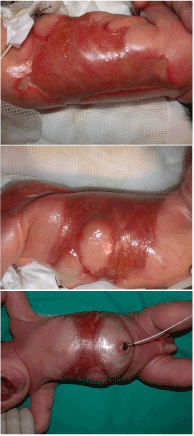
Figure 1. Skin defects at birth over back, flanks and abdomen.
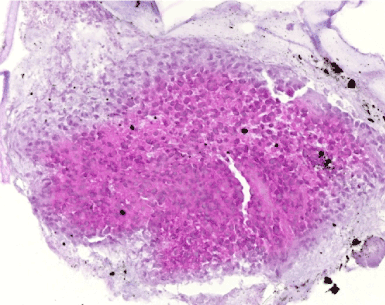
Figure 2. Histological analysis of a biopsy of an affected area. Hematoxiline-eosine coloration. No true epithelium layer is visible while stromal connective tissue with island of epithelial cells are present.
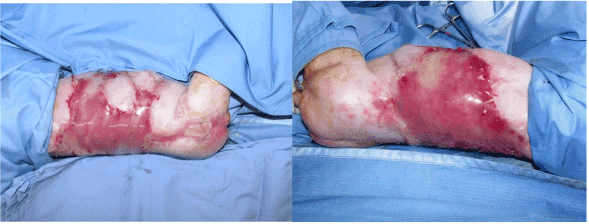
Figure 3. IDRT graft over flanks and abdomen.
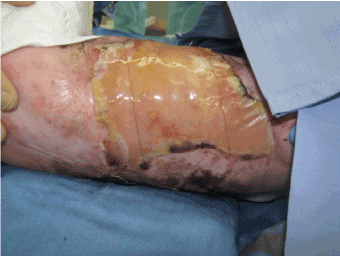
Figure 4. IDRT graft take: a peach color appears in area of regenerated dermal tissue
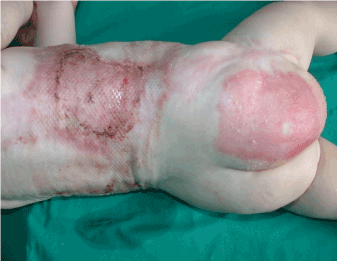
Figure 5. Split thickness skin graft and donor site after infection on the right side of the thorax
When examining the scars one year after the surgery, soft skin with great pliability was found where IDRT graft succeeded. After two years, growth and development of the patient were optimal and a soft pliable skin was present where Integra was used with success. On areas where a spontaneous healing occurred, fibrous band were observed as seen on the thigh (Figure 6).
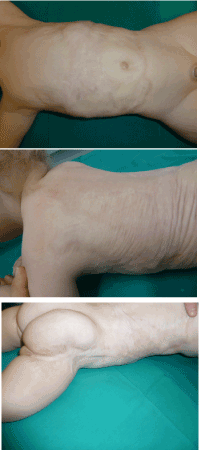
Figure 6. Scars appearance at 2 years. Softness and pliability of the scar where IDRT and skin graft were used (Top and Center). Fibrous band in the sites healed spontaneously (Bottom)
From the literature we know that there could be different consequences on the survival twin related to the time of foetus papyraceous intrauterine death. If the fetus dies during the first trimester the outcome of the viable twin is less severe than if the death happens during the following six months of pregnancy. In the latter case the newborn may develop extensive aplasia cutis or cerebral complications, such as infarction or palsy. In our case the fetus died at 12th week of pregnancy.
Maternal underlying diseases are also investigated for their possible correlation with ACC in foetus papyraceous. In the literature most of the mothers did not have any underlying disease known to disturb fetal growth. The mother of the present case was 32 years old, negative for Hepatitis C Virus, Hepatitis B Surface Antigen, and Cytomegalovirus. She had no history for co-morbidities like herpes simplex infections or varicella-zoster, or medications taken during pregnancy. The family history was negative for ACC as well.
To our knowledge, this is the first report in the literature of a Group V ACC case treated using IDRT. One case report dated from 2000 [9] reported a successful graft of skin defects with Alloderm for a comparable case. However, in this case, the substitute graft and the keratinocytes implantation were carried out during a single procedure, thus requiring the final surgery to be delayed until keratinocytes were available.
2021 Copyright OAT. All rights reserv
In conclusion, in newborns with extended ACC, we think that use of IDRT followed by skin graft of the defects is a good alternative to obtain good quality scars, especially compared to conservative treatment which could ultimately lead to contractile scars and subsequent secondary reconstructions.
This study was conducted without external funding. Authors thank Dr. Rosanna Quattrin for her contribution to review the manuscript.
- Tempark T, Shwayder TA (2012) Aplasia cutis congenita with fetus papyraceus: report and review of the literature. Int J Dermatol 51: 1419-1426. [Crossref]
- Frieden IJ (1986) Aplasia cutis congenita: a clinical review and proposal for classification. J Am Acad Dermatol 14: 646-660. [Crossref]
- Cambiaghi S, Schiera A, Tasin L, Gelmetti C (2001) Aplasia cutis congenita in surviving co-twins: four unrelated cases. Pediatr Dermatol 18: 511-515. [Crossref]
- Mannino FL, Jones KL, Benirschke K (1977) Congenital skin defects and fetus papyraceus. J Pediatr 91: 559-564. [Crossref]
- Schaffer JV, Popiolek DA, Orlow SJ (2008) Symmetric truncal aplasia cutis congenita following multifetal reduction of a sextuplet pregnancy. J Pediatr 153: 860-863.
- Pharoah PO (2009) Multiple births and aplasia cutis. J Pediatr 155: 598. [Crossref]
- Fusi L, McParland P, Fisk N, Nicolini U, Wigglesworth J (1991) Acute twin-twin transfusion: a possible mechanism for brain-damaged survivors after intrauterine death of a monochorionic twin. Obstet Gynecol 78: 517-520. [Crossref]
- Markman L, Sugar L, Zuker RM (1982) Association of aplasia cutis congenita and fetus papyraceus in a triplet pregnancy. Aust Paediatr J 18: 294-296. [Crossref]
- Simman R, Priebe CJ Jr, Simon M (2000) Reconstruction of aplasia cutis congenita of the trunk in a newborn infant using acellular allogenic dermal graft and cultured epithelial autografts. Ann Plast Surg 44: 451-454. [Crossref]






