Employment of a specific technology that generates point durations for physiologically patterned magnetic fields has produced reliably, robust analgesia in rodents and invertebrates that is equivalent in magnitude to clinical dosages of morphine. However morphine has been reported to facilitate oncogenesis and metastasis. To compare the treatments human breast cells were exposed to either morphine or to the analgesia-producing magnetic field pattern. Molecular assays demonstrated that both treatments affected mu-receptor opiate activity that could be blocked by classic antagonists. Whereas the morphine-treated cells displayed activation of molecular pathways that could encourage cell proliferation this profile was not observed in the magnetic-field treated cells. These results suggest that the appropriately applied magnetic field pattern could produce the desired clinical analgesia for some cancer patients but with reduced risk of facilitating metastases.
morphine, breast cancer cells, magnetic field analgesia, metastasis
Increased reports of chronic pain and reduction in quality of life are the most common consequences of the final stages of proliferation of cancer cells within the human body. During these stages the most common class of medications to attenuate the negative affect and nociception has been morphine or its derivatives. However there are reports that administration of morphine can facilitate metastasis [1]. Until recently these probabilities were considered of minimum consequence because the patient’s demise was considered imminent. There is now a tacit anticipation that more effective treatments for late stages for many cancers may facilitate an unexpected longevity. The treatment of pain by a pharmacological agent that could promote cancer development would be counterproductive.
As reviewed by Del Seppia et al. [2]. weak (microTesla to milliTesla) time-varying magnetic fields within the extremely low frequency range (1 to 1000 Hz) have been shown to produce elevations in nociceptive thresholds (“analgesia”) for a variety of species that include vertebrates and invertebrates. Fleming et al. [3] found that normal and brain-damaged rats, secondary to lithium-pilocarpine-induced seizures, displayed elevated threshold responses to nociceptive stimulation from electric current when exposed for only 20 min to a burst-firing (accelerating frequency modulated) magnetic field that was activated for 1 s every 4 s. The primary spectral densities were within the 10 to 40 Hz range.
Later Martin et al. [4] found that rats exposed to only 30 min of a burst-firing patterned field exhibited elevated nociceptive thresholds to thermal stimulation that was equivalent to or more effective than 4 mg/kg of morphine sulphate. The magnetic field exposure-induced analgesia could be blocked by pre-administration of the μ opiate antagonist naloxone. Whereas the rats habituated to the effects of both naloxone and morphine their analgesic responses did not habituate to the three daily magnetic field exposures. Martin and Persinger [5,6] isolated the effective parameters to produce analgesia and found that rats in which histologically-verified brain damage had been induced by lithium pilocarpine-induced seizures exhibited maintained elevations in nociceptive thresholds after three successive days of treatment. This was not apparent in normal rats.
Patients who had sustained closed head injuries and were experiencing clinically and psychometrically-evident depression and pain responded positively to transcerebral application (1 to 5 μT) of a burst-firing pattern magnetic field that was presented for 1 second every 3 or 4 seconds [7,8]. The effect size was comparable to those reported for TMS (Transcranial Magnetic Stimulation) that involves field strengths one million times more intense [9,10]. The rationale for these marked intensity differences have been explained by two different biophysical processes [11].
Some of our patients who had sustained chronic pain for years reported elimination of the discomfort after only three, weekly sessions, although the clinical protocol involved 6, weekly sessions. Healthy volunteers also reported diminished scores for psychometric inferences of pain and depression for this patterned field specifically but not to other patterns [12]. In general treatments once per week were more permanently effective than daily treatments [13]. Of approximately 1000 volunteers who have been exposed to variations of the burst-firing magnetic field the majority have experienced pleasantness as indicated by post-exposure ratings. The very small percentages (about 4%) who find it aversive also reported negative experiences or responses to morphine.
Considering the importance of developing procedures for increasing quality of life and reducing pain but also “to do no harm” we have been developing alternative protocols that might be beneficial for patients who experience chronic pain. The first question was to answer the question: if the data indicate exposure to appropriately patterned magnetic fields produces similar levels of analgesia as morphine derivatives but the later can increase the probability of metastases, are magnetic field exposures less oncogenic? Here we present evidence from cell culture that both morphine and patterned magnetic fields affect the μ receptors of human breast cancer cells but magnetic field exposures, unlike morphine, no not initiate the signalling pathways associated with cell proliferation.
Medium plates (~5x1066 cells) of human breast adenocarcinoma (MCF-7) cells, obtained from the cell bank ATCC and cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Gaithersburg, MD, USA) were exposed to one of two groups, namely a patterned magnetic field (Burst X) or chemical compound (morphine sulphate) to compare the efficacy in eliciting an opioid specific response. The overall treatment conditions were: 1: morphine (1 μM), 2: DAMGO (1 μM), 3: naloxone (1 μM), 4: naltrexone and (1 μM), 5: media only in the sham field (no magnetic field) condition and 6: morphine (1 μM), 7: DAMGO (1μM), 8: naloxone (1 μM), 9: naltrexone (1 μM) and 10: media only in the magnetic field exposed condition. All experiments were replicated in triplicate.
Chemical exposures
The opioid agonist, morphine sulphate (salt solution) was obtained from Sigma Aldrich. Cells were exposed to three concentrations, low (1 μM), medium (10 μM) and high (100 μM) to observe any changes in the expression of the mu-opioid and delta-opioid receptors. To further show the distinction between EMF and morphine induced effects upon the mu-opioid receptor, naloxone and naltrexone, two competitive antagonists with a high affinity u-opioid receptor and DAMGO, a synthetic opioid peptide with high μ-opioid receptor specificity were used for negative and positive controls respectively, against EMF/morphine. These compounds were used in concentrations of 1 μM, based on previously reported concentrations in cellular/opioid studies. In all cases, the compounds were dissolved at the appropriate concentration in fresh culture medium. Cells were incubated at 37°C for 24 or 35 hours before extracted for immunoblotting analysis.
Magnetic field exposures
The same numbers of plates were exposed to a magnetic field pattern, (“Burst-X”) which is known to elicit a similar in vitro analgesic response in rats to those who were injected with 4 mg/kg of morphine. A diagram of this pattern has been recently published by Murugan and Persinger [14]. The same numbers of cells exposed to the chemical exposures were seeded in fresh culture media and placed into a Helmholtz coil. The coil was 32.5x32.5x41 cm and was constructed from 305 m of 28 AWG wire (30 ohms) within about 235 turns. The strength of the field in the exposure area was ~5 microTesla. The magnetic field apparatus was similar to that found to produce changes in planarian behaviour [14]. The magnetic field was applied for 24 or 35 hours before extraction for immunoblotting analysis.
The magnetic field parameters were chosen based on the effectiveness in the treatment of pain, such as the type reported by patients who are diagnosed with depression and in rats that displayed thermal analgesia equivalent to a morphine injection. Our magnetic fields are generated by a different technology than function generators. Using specific computer software numbers between 0 and 256 generated from computers are transformed to between -5 to +5 V (127=0 V). A digital to analogue converter allows the conversion to current that is applied through the coil. Critical temporal and intensity parameters are programmable. The duration of each number between 0 and 256 (and hence voltage) is the point duration. The Burst-X pattern is composed of 230 numbers. The optimal point duration based upon many other studies has been 3ms. This value was applied here. Hence the duration of a single pattern is 690 ms. The time between each pattern was 3,000ms (3s), which is the optimal inter-stimulus-interval to produce maximum analgesia in rodents.
Molecular procedures
After exposures the cells were extracted. Cell lysates were obtained and subjected to traditional immunoblotting methods. The antibodies that were used were ERK, MEK, MOR1, DOR1, p53 and NF-B and caspase-3. All antibodies were obtained from Santa Cruz.
The first observation was that MCF-7 cells expressed a statistically significant higher concentration of the mu-opioid receptor (MOR-1) than that of delta opioid receptor (DOR-1) and these levels were increased (same strength of increase) following exposures to either morphine or the burst-X magnetic field pattern. In other words, the concentrations of MOR-1 expression increased with increasing exposure/doses of EMF or morphine. We observed the same increase with the positive control DAMGO.
In terms of signalling molecules, the results showed that both opioid agonists displayed the ability to increase the expression of signalling molecules (ERK and MEK) and of an apoptosis protein, (caspases-3). Interestingly the same expression of molecules was seen with Burst-X exposed cells (with a “doubling” as defined by the thickness of the band) when both agonist and Burst-X exposed together, in all proteins except caspases-3. In addition, cells exposed to Burst-X maintained higher expression of MOR-1 receptors longer (+24 hrs) than their chemical counterparts.
We also investigated the expression of a cell cycle regulation and signaling molecule p53, as well as a protein complex, NF-κB, that is increased when a cell is stressed. The results are shown in Figures 1 and 2. There was no change in NF-κB expression, suggesting that Burst-X exposure for 12 hours did not “stress” the cells (Figure 1) and increased the regulation in its cell cycle along with expression other proteins. This was not observed for cells that were exposed to morphine only (Figure 2). The numbers refer to the treatments. They are: 6 (control plus high concentration of morphine), 7 (control plus low concentration of morphine), 8 (EMF plus media only), 9 (EMF plus high concentration of morphine) and 10 (EMF plus low concentration of morphine).
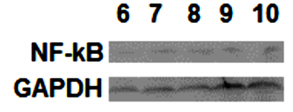
Figure 1: NFB expression is not changed with respect to treatment condition (see text for identification of lanes 6 through 10).
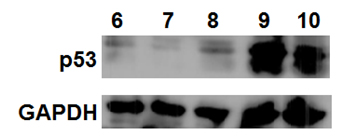
Figure 2: Differential changes are seen within adenocarcinoma cells as a function of treatment condition (see text for identification of treatments for lanes 6 through 10).
The differential proliferation was clearly evident after 48 hrs of exposure to the morphine or magnetic field treatments when standard confluence occurred. Cell counts were obtained under light microscopy through a haemocytometer after staining with Trypan Blue. As can be seen in Figure 3, there was a clear difference in average cell counts (SEM=Standard Error of the Mean), the inferential of proliferation, between the treatments which explained 77% of the variance [F(2,47)=75.95, p < . 001]. The breast cancer cells receiving the morphine exhibited significantly greater numbers compared to the breast cancer cells that received the Burst-X magnetic field treatment. These numbers did not differ significantly from the breast cancer cells exposed to the sham field condition.
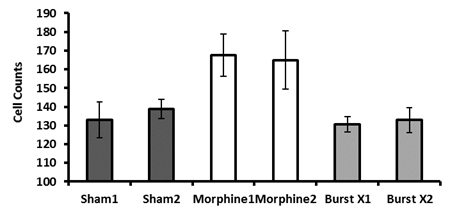
Figure 3: Number of cells per condition. Cells counts were conducted using a dilution in 500uL PBS. Means and SEMs, P < 0.05 are presented using ANOVA and Tukey’s post hoc analysis. 1 and 2 refers to different experiments
To our knowledge this is the first experimental demonstration that demonstrates that exposure to the same weak, physiologically patterned magnetic fields that promote analgesia-like behaviours in rats and planarian and either analgesia or persistent relief of chronic pain in human patients affects μ opiate receptor activity but does not recruit the signal molecules that can promote cell proliferation. On the other hand although morphine, as would be expected, markedly influenced the μ opiate receptor activity it also facilitated pathways that could contribute to cell proliferation, cell division, and potentially metastasis. These results support the reports of other researchers [1] that systemic application of morphine can promote metastasis. That the μ receptor was directly involved is indicated by the diminishment of the effects when classic opiate antagonists were first administered before the treatments.
The results are consistent with the inferences derived by Del Seppia et al. [2]. The results also compliment the innovative research by Alex Thomas [15] and his colleagues who were one of the first groups to review and to consider the cytoprotective and repair-capacity effects from brief exposures to extremely low frequency magnetic fields. Recently Karbowski et al. [16] found that the digital transformation of an actual quantitative electroencephalographic (QEEG) pattern from the anomalous region of a man whose presence affected cell activity evoked diminished cell proliferation of melanoma mouse cells when the pattern was applied as a magnetic field. The point duration of those fields was 3ms. Digitally-transformed QEEG patterns from the more normal patterns of that man’s brain did not affect the cell proliferation. These results suggest that individualized patterned magnetic fields could be developed for patients and hence increase the efficacy of the analgesia. This salience of this suggestion becomes significant when the emerging synergisms between pharmacological compounds and physiologically-patterned magnetic fields are considered for the 21st century [17].
We thank Dr. Robert M. Lafrenie for his advice and the use of his equipment during components of this study.
2021 Copyright OAT. All rights reserv



