Studies have suggested that silver nanoparticles can augment the cellular immune responses of cells and thus may contribute to immunopathology, especially as an autoinflammatory disorder. This study examines the interaction of silver nanoparticles with a co-culture of alveolar macrophages and lung epithelial cells to determine if the nanoparticles are capable of activating the inflammasome for sustained production of the pro-inflammatory cytokine interleukin-1beta. It is demonstrated here that interleukin-1beta is upregulated, both the transcript and the mature protein, following 24 hour exposure to 10 nm silver, but that caspase-1 function is downregulated, which prevents confirmation of the inflammasome activity. However, significant upregulation of interleukin-1beta is the contributing factor to many autoinflammatory disorders, indicating that prolonged exposure to silver nanoparticles, even at sub-lethal doses, could potentially cause pathological damage to the host through inflammation.
inflammasome, autoinflammatory disorder, pathogen-associated molecular patterns, chemokines, microbicidal, anti-microbial
Inflammasomes are large intracellular multiprotein complexes that assemble in the cytoplasm of innate immune cells, such as macrophages and dendritic cells. They respond to a large range of pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs) by promoting the secretion of pro-inflammatory cytokines such as interleukin 1beta (IL- 1β). So far, four inflammasomes have been identified, containing an inflammasome sensor, the adaptor protein apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (ASC-CARD), and pro-caspase-1. Inflammasome activation is crucial for host defense against pathogens but excessive or prolonged-activation, or loss of function, has been linked to autoinflammatory disorders, so a better understanding of toxicity associated with inflammasome activation is imperative for the prevention of autoimmune disorders, such as chronic asthma, inflammatory bowel disease (IBD), and many others [1-5].
Asthma is a chronic inflammatory disease characterized by airway inflammation, airway hyper-responsiveness and airway remodeling [2]. The inflammasome is activated by PAMPs and DAMPs, which are common exposure particulates known to intensify asthmatic symptoms. IL-1β activation initiates downstream signaling pathways and stimulates pro-inflammatory processes in the airway, leading to airway constriction, pain, and labored breathing. IL-1β also has the ability to promote the production of other cytokines and chemokines at the site of inflammation, further exacerbating the pathology [2].
Nanomedicine is an emerging field of science that involves the fabrication of nanomaterials that are designed to perform a specific function when added to a biological system [6-8]. Silver nanoparticles (Ag-NPs) have attracted the most interest of all nanoparticles due to their anti-microbial properties, high electrical conductivity, and optical properties [9]. One of the most desirable attributes of Ag-NPs is their ability to be a broad-spectrum microbicidal compound, killing a wide variety of bacteria, viruses, parasites, and fungi [9]. In spite of their incredible potential, Ag-NPs toxicological properties and potential negative human and/or environmental impact remains uncertain. Developing a greater foundation into the knowledge of the biological interactions of nanoparticles with humans, and any potential negative ramifications following exposure is vital for the successful application of Ag-NPs. A zebrafish model demonstrated that the genes encoding caspases and IL-1β can be upregulated following exposure to Ag-NPs, suggesting that Ag-NPs may interfere with the activity of the inflammasome [10]. Since nanoparticles are also being utilized in the treatment of autoinflammatory disorders, such as asthma, it is important to determine if the nanoparticles themselves may exacerbate symptoms [11]. If inflammasome activation occurs with exposure to nanoparticles, then prolonged treatment, or repeated inhalation exposure during the manufacturing process, could result in the development or worsening of chronic asthma, resulting in a debilitating condition.
The aim of this study was to examine whether or not Ag-NPs were capable of activating the human inflammasome NLRP-3 and to determine if IL-1β is upregulated in response to Ag-NP exposure. The hypotheses were tested using a previously described macrophage-pneumocyte co-culture system that was designed to more accurately mimic the lung microenvironment [12]. Using two cell lines concurrently has been demonstrated to be a more accurate model for nanoparticle toxicity since it allows for the intercellular cross-talk necessary for the establishment of an effective immune response [12].
Nanoparticles
10 nm Biopure Ag-NPs were purchased from nanoComposix Inc (San Diego, CA). The Ag-NPs had a size distribution of 7-15 nm as determined via transmission electron microscopy by the manufacturer. The Ag-NPs exhibited an optical density of 125 cm-1 at a peak wavelength of 395 nm, which too was determined by the manufacture.
Human Lung Co-cultures
The A549 cell line, human lung epithelial cells, and U937 cell line, human alveolar macrophages, were chosen as an in vitro model to mimic the lung environment [12]. Both cell lines are ideal cell types for immune function studies since intercellular communication is critical in tissues, and phagocytic cell function is necessary for studying the inflammasome response [12]. Both A549 and U937 cell lines were obtained from the American Type Culture Collection (ATCC, CCL-185 and CRL-1593.2, respectively) and grown in RPMI-1640 media supplemented with 1% Penicillin-Streptomycin, 1% L-glutamine and 10% heat-inactivated fetal bovine serum (FBS). The U937 cells were stimulated with 100 ng/mL of phorbol-12 myristatte-13-acetate (PMA) for 48 hours to induce differentiation from monocytes into macrophages [13,14]. The A549 epithelial cells were then co-cultured with U937 macrophage cells in a 3:1 (A549:U937) ratio, and then co-cultured cells were seeded in a 96 well or a 12 well plate at densities of 1x105 or 1x106 total cells per well, respectively. Twenty four hours later the co-cultures were dosed with the NPs and used for the following assays [12].
Cell Viability/Cytotoxicity
The A549 and U937 co-cultured cells plated in the 96 well plate were treated with increasing doses (0-100 µg/mL) of Ag-NPs diluted in serum-free cell culture media to determine cytotoxicity. After 24 hours incubated at 37°C with 5% CO2 the cell viability was assessed using the CellTiter 96 AQueous One (MTS) kit from Promega (Madison, WI), which measures mitochondrial function. The MTS kit was used per manufacturer’s instructions and was read using the BioTek ELx800 (BioTek Inc, Winooski, VT) with optical density determination at a light wavelength of 490 nm.
RNA purification & quantification
The 12 well plates were treated with Ag-NPs at a concentration of 0, 10, or 50 µg/mL diluted in serum-free cell culture media and were incubated at 37°C with 5% CO2 for 24 hours. Following the incubation, the cell culture supernatant was removed and used for the ELISA, while the resulting cells were used for RNA isolation for gene expression studies. The RNA was extracted using a modified Trireagent (Sigma Aldrich, St. Louis, MO) protocol. Briefly, the cell supernatant was removed, the cells were washed twice, and 350 μL of Trireagent was added to each well. The lysate was transferred to a sterile microcentrifuge tube and vortexed for 2 minutes. The cellular debris was removed by centrifuging at 12,000 x g for 1 minute in a Beckman Coulter Microfuge 22R Centrifuge (Irving, TX). The resulting supernatant was added to 350 μL of 100% ethanol mixed before the contents were transferred to a spin column with 2 mL collection tube (EconoSpin, Epoch Life Science, Missouri City, TX) and centrifuged at 2000 x g for 1 minute discarding the flow through. The spin columns were washed with first 700 μL of a High Stringency Wash Buffer (2M guanidine thiocyanate; 1% v/v sodium dodecyl sulfate; 55% ethanol) and then 700 μL of Low Stringency Wash Buffer (80% ethanol), centrifuging each for 2000 x g for 1 minute and discarding the flow through. The spin column was dried by centrifuging again at 2000 x g for 1 minute with no buffer. The spin column was transferred to a 1.5 ml collection tube and 50 μL of RNase free water was added to the membrane for RNA extraction at 2000 x g for 30 seconds. The flow through was stored at -80°C until needed. A DNase digestion was performed (RQ1 RNase-free DNase, Promega, Madison, WI) and RNA was quantified using the Qubit RNA HS Assay kit by Invitrogen (Carlsbad, CA) as per the manufacturer’s instructions.
qRT-PCRs
Extracted RNA was reverse transcribed to cDNA using the Quanta qScript cDNA SuperMix as per the manufacturer’s instructions (Beverly, MA). One μL of the resulting cDNA was used to perform quantitative real time PCR (qRT-PCR) using the PerfeCTa SYBR Green SuperMix Reagent (Quanta Biosciences, Beverly, MA) and 0.5 μL of primer mix (0.2 μM of each primer) for human-IL-1β with GADPH (Table 2). Each cDNA sample was prepared in triplicate with each corresponding primer set listed in Table 1 and loaded into a 384-well PCR plate for analysis in the Roche 480 Light Cycler (Basel, Switzerland) to determine the threshold cycle of each sample. The following cycles were used for DNA amplification: 1 minute denature at 95°C; 45 cycles of 10 seconds at 95°C, 30 seconds at 60°C with a quantification fluorescence read, and 30 seconds at 72°C; followed by a melt curve of 10 seconds at 95°C, 30 seconds at 40°C with continuous fluorescence readings to 95°C; and a final cool down to 40°C. The threshold cycles were used to determine the fold change using the delta-delta CT method [15].
Table 1. DNA primers for mouse tissue qRT-PCR (human IL-1β).
Gene |
Forward Primer |
Reverse Primer |
Human IL-1β |
GGAGAATGACCTGAGCACCT |
GGAGGTGGAGAGCTTTCAGT |
Human GADPH |
TTGCCATCAATGACCCCTTC |
ATCTCGCTCCTGGAAGATGG |
ELISAs
The supernatants from the 12 well plates were used to test for IL-1β protein production using the human Ready-Set-Go ELISA kit from Affymetrix (Santa Clara, CA) according to manufacturer’s protocols. This procedure utilized the optional stop solution (2N H2SO4) and was read in the BioTek ELx800 (BioTek Inc, Winooski, VT) with optical density determination at a light wavelength of 450 nm.
Caspase-1 Activity
The A549 and U937 co-cultured cells lines seeded in the 96 well plate were treated with 0, 10, 25, or 50 μg/mL of Ag-NPs for 24 h, and then caspase activity was assessed using the Caspase-1 Fluorometric Assay Kit from PromoKine (Heidelberg, Germany). The Caspase-1 kit was used per manufacturer’s instructions and was analyzed using the BioTek Flx800 (Winooski, VT) with an excitation/emission of 400nm/505nm.
Statistical analysis
All statistical analyses performed compared the presence and the absence of nanoparticles and utilized multiple Student’s t-tests for determination of significant differences between experimental values and controls with p < 0.05 indicating that treatments were significantly different from control.
Mitochondrial function
There were no significant decreases in mitochondrial function in co-cultured cells when dosed with two-fold dilutions from 100-0 µg/mL of Ag-NPs, indicating that the Ag-NPs were not cytotoxic to the co-culture. A reduction to 84.5 % cell viability was observed at Ag- NP concentrations of 100 µg/mL (Figure 1), but due to some variability in the assay, there were no statistically significant cytotoxic effects on mitochondrial function at any dose (Figure 1).
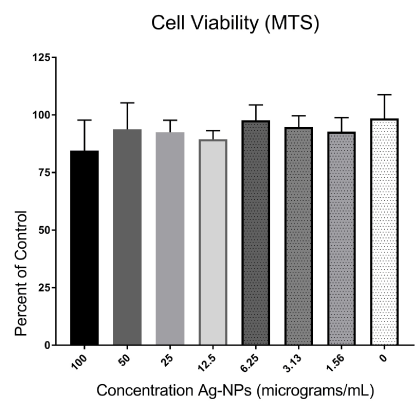
Figure 1. Cytotoxic effect of the Ag-NPs in A549/U937 co-cultures. Co-cultured cells were treated with Ag-NPs at two-fold dilutions from 100-0 μg/mL. Following 24-hours of exposure, the cells were assessed for cell viability using an MTS mitochondrial function assay with the optical density values used to determine the percent of control (0μg/mL) following nanoparticle exposure.
Interleukin-1beta transcription
IL-1β gene expression was significantly up-regulated in cells following a 24-hour exposure to Ag-NPs at concentrations of 10 and 50 μg/mL compared to the control sample (0 μg/ml) (Figure 2). The gene GADPH was used as an internal control to normalize RNA in the samples.
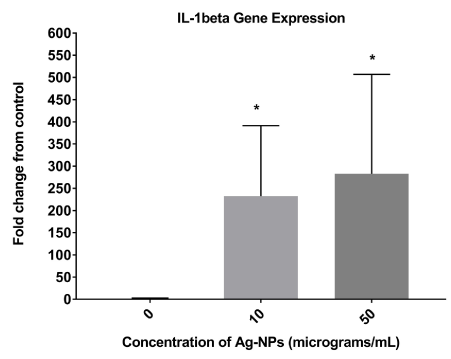
Figure 2. Relative IL-1β gene expression in Ag-NP-treated cells. Co-cultured cells were treated with Ag-NPs at various concentrations (0, 10, 50 μg/mL). Following 24-hours of exposure, the supernatants were assessed for IL-1β gene expression with the optical density values used to determine the percent of control (0 μg/mL) following nanoparticle exposure. * denotes statistically significant with p < 0.05.
Interleukin-1beta translation
IL-1β protein production was slightly increased following a 24-hour exposure of 10 μg/mL of Ag-NPs, but was significantly upregulated at 25 and 50 μg/mL as compared to the control (0 μg/mL) (Figure 3).
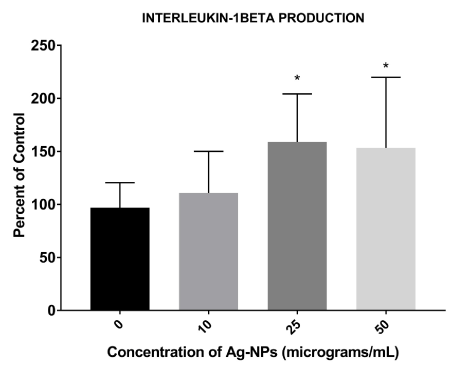
Figure 3. Relative IL-1β protein expression in Ag-NP-treated cells. Co-cultured cells were treated with Ag-NPs at various concentrations (10, 25, 50 μg/mL). Following 24-hours of exposure, the supernatants were assessed for IL-1β protein levels with the optical density values used to determine the percent of control (0 μg/mL) following nanoparticle exposure. * denotes statistically significant with p < 0.05.
Caspase-1 activation
There is a slight basal activation of caspase activity in untreated cells, as they were significantly higher than the cell-free reagent control (Figure 4). Since the Ag-NP-treated cells were actually below the reagent control, it is hypothesized that the nanoparticles themselves are interfering with the fluorescence in this assay (Figure 4). However, an increased caspase-1 activation is not observed as hypothesized, but instead an approximately 80% reduction in activity occurred regardless of dose (Figure 4). This decrease was not statistically significant from control at any dose as determined by Student’s T-tests.
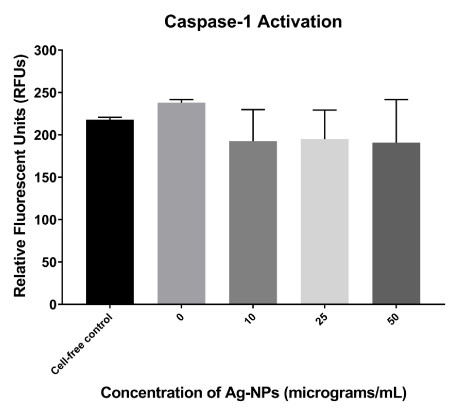
Figure 4. Relative caspase-1 activity in Ag-NP-treated cells. Co-cultured cells were treated with Ag-NPs at various concentrations (10, 25, 50 μg/mL). Following 24-hours of exposure, the supernatants were assessed for caspase-1 expressed in RFUs. A reagent control for background fluorescence was used in the absence of any cells.
The overall aim of this study was to examine the inflammatory activation of Ag-NPs with A549/U937 co-cultured cells to understand the potential negative impact Ag-NPs may or may not have on inflammatory disorders. In spite of their incredible potential, Ag-NPs impacts on cell function, especially at sub-lethal doses, remains uncertain. Developing a greater foundation into the knowledge of the biological interactions of nanoparticles with humans and the negative ramifications, if any, is vital for the successful application of Ag-NPs as therapeutics. This study was designed to mimic the lung microenvironment utilizing human A549/U937 co-culture cell lines to determine if the inflammasome is indeed being activated by exposure to nanoparticles to determine if they should be used for therapeutic applications. Even though the A549/U937 co-cultured cells did not exhibit cell death as a result of exposure to Ag-NPs, the qRT-PCR and ELISA assays did demonstrate changes in gene and protein expression modification, specifically the up-regulation of IL-1β.
The upregulation of IL-1β suggests that Ag-NPs are having an impact on cellular transcription and translation, which could augment cellular function even at “non-toxic” doses. It is hypothesized that the upregulation of IL-1β is occurring through the activation of the inflammasome, however it could not be concluded since the corresponding upregulation of caspase-1 was not observed. However, caspase-1 activity is thought to be essential in the proteolytic cleavage of pro-IL-1β to its active form (IL-1β), which leads to speculation that the Ag-NPs may just be interfering with the fluorometric readings. Caspase-1 exists in unstimulated cells as a catalytically inactive precursor pro-caspase -1. The ASC-CARD domain of the inflammasome is responsible for the recruitment of pro-caspase-1 leading to caspase-1 oligomerization and auto-proteolytic conversion of the pro-enzyme into its active form [16]. Activated caspase-1 elicits the maturation and secretion of the pro-inflammatory cytokine IL-1β, resulting in subsequent immune responses [16-17]. If there is indeed a 20% down regulation of caspase-1 by exposure to Ag-NPs, this decrease may not be physiologically significant enough to impact the biological processing of IL-1β since an increase in protein production was observed, and it was all in a secreted form indicating that it is an active cytokine. The down-regulation of caspase-1 could be due to Ag-NPs ability to inhibit enzyme activity, which has been shown with various enzymes in previous studies [18]. Regardless, there appears to be enough caspase-1 activity to activate IL-1β, and caspase-1 activation followed by subsequent IL-1β production, leads to pyroptosis, which is an inflammatory-linked cell death. Pyroptosis is characterized by rapid plasma-membrane rupture, DNA fragmentation, and the release of pro-inflammatory cytosolic contents into the extracellular space, which could potentially lead to destruction of neighboring cells [19].
An up-regulation of IL-1β results in uncontrolled cellular signalling leading to continuous cytokine and chemokine production at the site of inflammation. Excessive inflammation will have an impact on the immune system’s ability to combat disease. Too much activation of the inflammatory response can be detrimental to the host, and is the main contributing factor to the pathological disorders referred to as autoinflammatory disorders. This study, along with others, demonstrate Ag-NPs ability to increase IL-1β production, suggesting that Ag-NPs may be capable of inflammasome activation [10,20]. However, this study, as well as previous ones that have shown IL-1β increase, are all single-exposure studies [10,20]. Therefore it is also important to assess continuous exposure to Ag-NPs, which could occur through manufacturing or therapeutic use, to determine the potential of Ag-NPs to cause chronic inflammation, and thus autoinflammatory disease.
To what extent are cellular transcription and translation being altered? How are Ag-NPs interfering with cellular function? These are questions that still remain in the field of nanotoxicology, and must be addressed prior to large-scale therapeutic use of these materials. In vitro studies have determined that the basal level of NLRP-3 in resting cells is not sufficient to activate the inflammasome. The disturbance of cellular homeostasis is the key to inflammasome assembly. It is understood that successful NLRP-3 inflammasome activation requires a two-checkpoint signal process. Priming signal is typically triggered by ligand binding to toll-like receptors (TLRs) or NOD-like receptors (NLRs). This results in activation of NF-κB, which translocates to the nucleus and activates the transcription of NLRP3 and pro-IL-1β [2,20]. A second activation signal generated by a wide range of exogenous and endogenous stimuli including an ion flux, phagosomal destabilization, mitochondrial reactive oxygen species (mtROS), or mitochondrial DAMPs, which will result in NLRP-3 inflammasome assembly [19,21-22]. Future studies need to focus on addressing how Ag-NPs can stimulate TLRs or NLRs, which typically respond to microbial pathogens.
It is imperative to fully understand any negative implications of Ag-NPs for therapeutic applications, but it has its difficulties since cell viability assays do not always depict altered cellular function. Ag-NPs appear to be activating the inflammasome of the cell, but how are they initiating the priming signal, which is thought to have to be a biological ligand? Regardless, they are activating the production of IL-1β at non-toxic doses, which could potentially lead to pathological consequences. Or perhaps, since inflammation is necessary for a successful immune response, it is possible that this activation may actually assist the body in successful removal of a pathogen if the Ag-NPs are used as an antimicrobial therapeutic, if the IL-1β levels are not sustained. However, it must be known if the upregulation of the inflammatory response is reversible, or if multiple exposures may lead to chronic autoinflammatory disorders. A broader understanding of the benefits and consequences of nano-based therapies is necessary for the use of these materials in the field of medicine, especially as it pertains to prolonged activation or repeat exposures.
- Ringel-Scaia V, McD2021 Copyright OAT. All rights reservs Conundrum: NLR Inflammasome Modulation of Gastrointestinal Inflammation during Inflammatory Bowel Disease. Crit Rev Immunol 36(4): 283-314. [Crossref]
- Lee S, Suh GY, Ryter SW, Choi A (2016) Regulation and Function of the Nucleotide Binding Domain Leucine-Rich Repeat-Containing Receptor, Pyrin Domain-Containing-3 In?ammasome in Lung Disease. Am J of Respir Cell and Mol Biol. 54(2): 151-160.
- Lee TH, Song HJ, Park CS (2014) Role of inflammasome activation in development and exacerbation of asthma. Asia Pac Allergy 4: 187-196. [Crossref]
- Park JH, Peyrin-Biroulet L, Eisenhut M, Shin JI (2017) IBD immunopathogenesis: A comprehensive review of inflammatory molecules. Autoimmun Rev 16: 416-426. [Crossref]
- Wachsmann P, Lamprecht A (2012) Polymeric Nanoparticles for the Selective Therapy of Inflammatory Bowel Disease. Methods Enzymol 508: 377-397. [Crossref]
- Braydich-Stolle LK, Hussain SM, Schlager JJ, Hofmann MC (2005) In-Vitro Cytotoxicity of Nanoparticles in Mammalian Germline Stem Cells. Toxciol Sci 88(2): 412-419. [Crossref]
- Petros RA, DeSimone JM (2010) Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov 9: 615-627. [Crossref]
- Wei L, Lu J, Xu H, Patel A, Chen ZS, et al. (2015) Silver nanoparticles: synthesis, properties, and therapeutic applications. Drug Discovery Today 20(5): 595-601. [Crossref]
- Tran QH, Nguyen VQ, Le AT (2013) Silver nanoparticles: synthesis, properties, toxicology, applications and perspectives. Advances in Natural Sciences: Nanoscience and Nanotechnology 4: 1-20.
- Speshock JL, Elrod N, Sadoski DK, Maurer E, Braydich-Stolle LK, et al. (2016) Differential organ toxicity in the adult zebra fish following exposure to acute sub-lethal doses of 10 nm silver nanoparticles. Front Nanosci Nanotech 2(3): 144-120.
- Smart CB, Yap WT, Neef TP, et al. (2016) Biodegradable antigen-associated PLG nanoparticles tolerize Th2-mediated allergic airway inflammation pre and postsensitization. Proc Natl Acad Sci USA 113(18): 5059-5064. [Crossref]
- Braydich-Stolle LK, Speshock JL, Castle A, Smith M, Murdock RC, et al. (2010) Nanosized aluminum altered immune function. ACS Nano 4: 3661-3670. [Crossref]
- Baek YS, Haas S, Hackstein H, Bein G, Hernandez-Santana M, et al. (2009) Identification of novel transcriptional regulators involved in macrophage differentiation and activation in U937 cells. BMC Immunology 10:18 1-15. [Crossref]
- Yang J, Zhang L, Yu C, Yang XF, Wang H (2014) Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res 2(1): 1-9. [Crossref]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402-408. [Crossref]
- Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, et al. (2004) NALP3 forms an IL-1ß-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 20: 319-325. [Crossref]
- Keller M, Rüegg A, Werner S, Beer HD (2008) Active caspase-1 is a regulator of unconventional protein secretion. Cell 132: 818-831. [Crossref]
- Speshock JL, Braydich-Stolle LK, Szymanski ER, Hussain SM (2011) Silver and Gold Nanoparticles Alter Cathepsin Activity In vitro. Nanoscale Res Lett 6: 17. [Crossref]
- Bergsbaken T, Fink SL, Cookson BT (2009) Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 7: 99-109. [Crossref]
- Elrod N, Brady J, Rathburn H, Speshock JL (2017) Alteration of Cytokine Profiles Inhibits Efficacy of Silver Nanoparticle-based Neutralization of arenaviruses. Toxicology: Open Access 3(2): 1-6
- Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, et al. (2009) Cutting edge: NF-?B activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 183: 787-791. [Crossref]
- Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, et al. (2006) Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440: 228-232. [Crossref]




