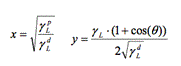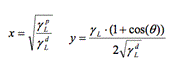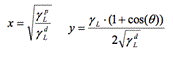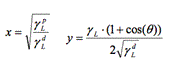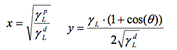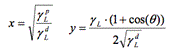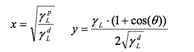The aims of this study were to evaluate the change in wettability and surface tension of metallic and polymeric surfaces before and after disinfection using current and new disinfectant products such as Bioxy. Seven materials often found in hospitals were used: Linoleum, vinyl, melamine, PVC, stainless steel, galvanized steel and aluminum. 7 disinfectants were used: Bioxy H1, Bioxy H5, Bioxy +1, Bioxy +5, sodium hypochlorite, hydrogen peroxide and quaternary ammonium. 24 samples of each of the 7 materials were used. The samples were divided into 3 groups: Water, formamide and diiodomethane. These 3 groups were subdivided into 8 groups as follows: original material, material + Bioxy H1, material + Bioxy H5, material + Bioxy +1, material + Bioxy +5, material + sodium hypochlorite, material + hydrogen peroxide and material + quaternary ammonium. We calculated surface tension with the Owens/Wendt model by using contact angle measurements using the sessile drop method. The results showed that the majority of the time Bioxy H5 was used (in some cases, Bioxy H1 as well), the contact angle was significantly lower, and surface tension was greater.
contact angle, disinfectant, surface tension, wettability
Abbreviations:
CA: contact angle; HP: hydrogen peroxide; PVC: polyvinyl chloride; QA: quaternary ammonium; SH: sodium hypochlorite; SV: Surface-vapor
Nosocomial infections are one the primary causes of infections in hospitals and health centers, and controlling it requires a better understanding of the disinfectants used. Nosocomial infections have an incidence rate of 5-10 % [1]. A WHO prevalence study puts its prevalence rate at 3.0-20.7%. By having infection control programs in hospitals, 33% of nosocomial infections can be prevented [1]. It has been reported that there is a quarter of a million nosocomial infections that occur annually [2]. Amongst the most common ones, infections caused by Staphylococcus epidermidis and Staphylococcus aureus represent 70-90% of the infections found in implants [3]. Moreover, it is important to elucidate whether these disinfectants interfere positively or negatively with the adhesion mechanism, as they can help determine which disinfectant is most suitable depending on the surface.
The purpose of this study was to compare the effects of the different disinfectants on the surface of various materials commonly found in the hospital environment. A complete study about how these products would affect hospital’s materials surface is determinant for its safe use as well as for its improvement. Economically, this project has huge impact at global market of hospital’s disinfection, and in some cases sterilization.
Our objective in the first part of our study was to determine the potential damaging effects of the 7 disinfectants on various materials. Our primary concern for this study is to establish the effect these disinfectants have on the wettability and surface tension of the same materials, to have a better understanding of the physiochemical properties of the materials. Moreover, surface roughness, hydrophobicity and surface free energy are known to influence bacterial adhesion [4,5]. In regards to surface tension, depending on the physicochemical properties of the substrate, the bacterial strain and the solution (or disinfectant) used, bacterial adhesion may vary greatly [6]. Hence, surface hydrophobicity is important in the interaction between surface’s composition and the bacteria.
The objective of the present study is therefore to test and compare each of the contact angles (wettability) of various surfaces commonly found in hospitals when in contact with the seven disinfectant agents. Wettability plays an important role surface cleaning and disinfection.
Bioxy H is a powder product that generate 3 disinfectants: quaternary ammonium (Quats), hydrogen peroxide and neutral peracetic-acid; in comparison with Bioxy+ which one does not contain the presence of ammonium compound. During this study is important to compare the effect of Bioxy products with the active compounds that are present also in products of the competitors, Sodium Hypochlorite (NaOCl), Hydrogen Peroxide (H2O2) and Quaternary ammonium (NR4+), in order to determine the effect of the surface of each reactive. Studies have shown that certain products have a different impact on cleansing ability and disinfection then others. Sodium hypochlorite for example has poor cleaning abilities even though it has the proper characteristics for a disinfectant [7].
In order to do so, Bioxy H and Bioxy + were each prepared at two concentrations, 1% and 5%, and were then compared to other disinfectants used in the hospital: sodium hypochlorite, hydrogen peroxide and quaternary ammonium. Each of these disinfectants were then tested on various materials commonly found in hospitals such as aluminum, galvanized steel, stainless steel, vinyl, linoleum, PVC and melamine.
One of the new products designed by ATOMES FD, Bioxy H, is composed of Sodium Carbonate (30-70%), ethylbenzyl ammonium chloride (2%) and chloride (2%), along with other non-hazardous components. This powered product was mixed with 100ml of sterile deionized water and was mixed for 10 minutes prior to testing.
Once Bioxy H dissolves in water, it releases three active compounds; peracetic acid, hydrogen peroxide and polyQuaternary ammonium chloride [8]. Peracetic acid is a compound known to sanitize surfaces [9]. We would like to analyze the effect of these compounds on the different surfaces.
Solution preparations
Bioxy H 1% concentration was prepared by mixing 100 ml H2O with 1g Bioxy H. for Bioxy H at 5% concentration, 100 ml H2O was mixed with 5g of Bioxy H product. The same proportions were applied for Bioxy +. At 1% concentration we mixed 100 ml H2O with 1g Bioxy+, and at 5% concentration we mixed 100 ml H2O with 5g Bioxy+. Sodium Hypochlorite (SH) at 10% (100 000ppm) was prepared by mixing 100ml of H2O with 4.16 ml SH. Hydrogen Peroxide (HP) at 30% (300 000ppm) was prepared by mixing 100ml of H2O with 3 ml of HP. Finally, for Quaternary Ammonium (QA) 10% (100 000ppm), 100 ml of H2O was mixed with 5 ml of QA.
Measurement of contact angle (Wettability)
The most common approach to determine the wettability of a surface is by the sessile drop technique using a goniometer. The wettability is quantified by measuring the contact angle (CA). Surfaces with CA between 0° to 90° are considered hydrophilic, while CA between 90° and 180° are considered hydrophobic [10]. Studies have shown that, as oppose to hydrophobic surfaces, hydrophilic surfaces are more likely to enhance cell adhesion, proliferation and differentiation [11,12]. The wettability is crucial for the evaluation of adhesion properties and for the analysis surface modification [13]. The more hydrophilic a surface is, the more easily a disinfectant will spread across the surface and disinfect it. However, as a tradeoff, the more a liquid spread, the more it can get absorbed by the surface and cause damaging effects. Theoretically speaking, hydrophobic bacterial strains (including hydrophobic cell membranes) are more likely to adhere to hydrophobic surfaces, and it goes for hydrophilic bacteria with hydrophilic surfaces [14]. For example, some studies have shown that there seems to be a correlation between bacteria such as S Staphylococcus aureus and S. epidermidis and hydrophobicity (surface wettability), and that it leads to its adhesion [15].
To measure the contact angle, and thereby wettability, we used a VCA Optima XE from AST Products, and a 100 µl syringe Hamilton Company. Each of the 7 materials was disinfected with each of the 7 disinfectant products (and one control) as follows: Original sample, Original + Bioxy H1, Original + Bioxy H5, Original + Bioxy +1, Original + Bioxy +5, Original + sodium hypochlorite, Original + hydrogen peroxide, Original + quaternary ammonium.
We rubbed each of the seven products 10 times in the same direction to disinfect every surface. They were then air dried for at least 20 minutes and placed on the contact angle equipment. The syringe was rinsed 3 times with water before use. Contact angle measurements were done using 2µl of distilled water on each sample. The measurements were taken ≈5 seconds after the droplet was placed on the surface to allow it to stabilize more with its environment. Three measurements of the contact angle were taken (for both the left and right side), and the average was calculated. We then calculated the standard deviation of each surface and performed a T-test to determine whether or not there was a statistical significant difference between the samples.
Measurement of surface tension
Surface tension measures the surface reactivity or adhesiveness to its environment [16]. Commonly, surface tension is caused by the asymmetry of the cohesive forces of molecules at a surface, which tends to minimize the surface area [17]. Disinfectant products, and subsequently bacteria, may adhere or not to the surface depending on the physicochemical properties of the substrate, bacterial strain and aqueous solution used [18]. In this study, the surface tension at the solid-vapor interface of each surface was calculated.
To measure surface tension, the same procedure as the one described above took place, however the procedure was repeated two more times using this time formamide and diiodomethane (as opposed to just water for wettability). The syringe was rinsed 3 times with each liquid respectively. The average of the contact angle measurement of each sample was then calculated. We used the Owen-Wendt model to calculate the surface-vapor tension (SV) [19].
This study evaluated the wettability and surface tension of 7 materials commonly found in hospitals that were disinfectant with 7 different disinfection products. Comparative data of contact angles showed in the Figures (Figures 1a to 7a) were subjected to statistical analysis. Corresponding surface tension are shown in Figure 1b to 7b respectively.
The results showed that the use of Bioxy H5, and sometimes Bioxy H1, increased the surface tension of the materials.
Linoleum
All the linoleum surfaces are considered hydrophilic since they have a contact angle smaller than 90°. Bioxy H5 is the most hydrophilic with an angle of 24.2° (Figure 1a). A T-test shows there was a significant difference between Bioxy H5 and the other disinfectants.
Wettability
(Keep Figure 1a here)
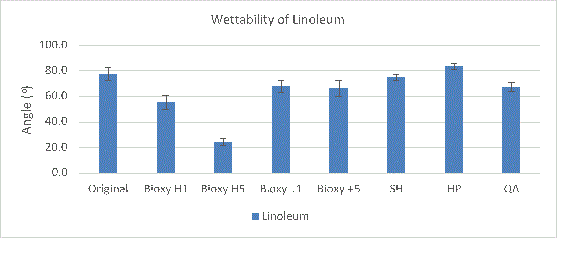
Figure1a. Wettability (contact angle) of linoleum with original surface and with the addition of seven disinfectants. 3 readings of each sample was taken (n=3).
Surface Tension
(Figure 1b here)
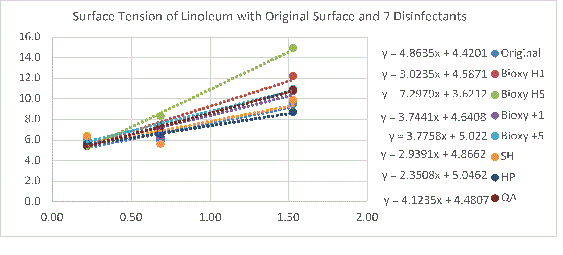
Figure 1b. Surface tension of linoleum with original surface and with the addition of the seven disinfectants. 3 readings of each sample(n=3) was taken with water, form amide and diazomethane.
Where
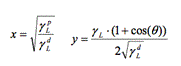
|
Original |
Bioxy H1 |
Bioxy H5 |
Bioxy +1 |
Bioxy +5 |
SH |
HP |
QA |
SV (mN/m) |
43,19 |
30,18 |
66,37 |
35,56 |
39,48 |
32,32 |
30,99 |
37,08 |
Vinyl
All the vinyl surfaces are more or less hydrophilic with an angle ranging between 12.73° and 90.7°. Bioxy H5 makes vinyl the most hydrophilic with an angle of 12.73° (Figure 2a). The T-test showed there was a significant difference between Bioxy H5 and the other products.
Wettability
(Figure 2a here)
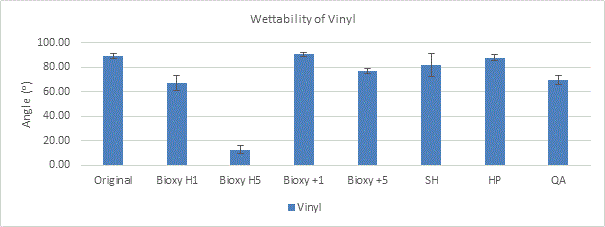
Figure 2a. Wettability (contact angle) of vinyl with original surface and with the addition of seven disinfectants. 3 readings of each sample was taken (n=3).
Surface Tension
(Figure 2b here)
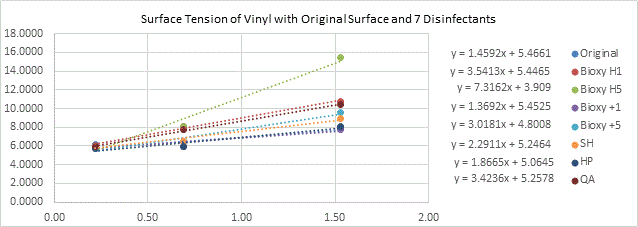
Figure 2b. Surface tension of vinyl with original surface and with the addition of the seven disinfectants. 3 readings of each sample(n=3) was taken with water, formamide and diiodomethane.
Where
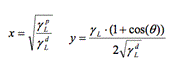
|
|
Original |
Bioxy H1 |
Bioxy H5 |
Bioxy +1 |
Bioxy +5 |
SH |
HP |
QA |
SV (mN/m) |
|
29,88 |
29,66 |
15,28 |
29,73 |
23,05 |
27,52 |
25,65 |
27,64 |
Melamine
Just like the original surface, all the disinfectants make melamine a hydrophilic surface. Bioxy H5 (47.3°) is statistically significantly lower than all the other surfaces except Bioxy H1. Since there was no statistical difference with Bioxy H1 (53.55°), more t-tests were done, and it was found that there is also a significant difference between Bioxy H1 and the other products.
Wettability
(Figure 3a here)
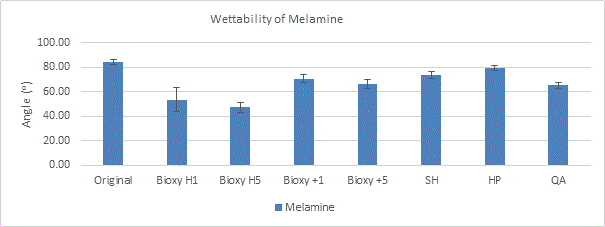
Figure 3a. Wettability (contact angle) of melamine with original surface and with the addition of seven disinfectants. 3 readings of each sample was taken (n=3).
Surface Tension
(Figure 3b here)
Figure 3b. Surface tension of melamine with original surface and with the addition of the seven disinfectants. 3 readings of each sample(n=3) was taken with water, formamide and diiodomethane.
Where
|
Original |
Bioxy H1 |
Bioxy H5 |
Bioxy +1 |
Bioxy +5 |
SH |
HP |
QA |
SV (mN/m) |
32,60 |
46,69 |
50,57 |
41,09 |
35,02 |
35,69 |
36,54 |
41,74 |
PVC
Bioxy +1 and Bioxy +5 are the only two disinfectants that seem to make PVC slightly hydrophobic, with angles of 91.52° and 92.93° respectively. T-tests were performed and it was observed that Bioxy H1 (14.37°) and Bioxy H5 (12.13°) were statistically significantly lower than the other disinfectants.
Wettability
(Figure 4a here)
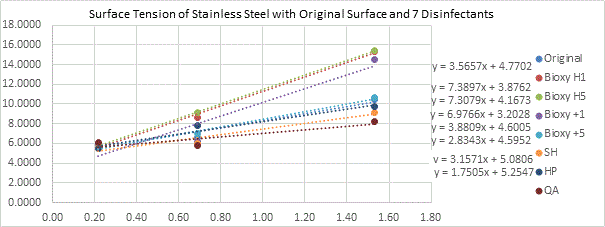
Figure 4a. Wettability (contact angle) of PVC with original surface and with the addition of seven disinfectants. 3 readings of each sample was taken (n=3).
Surface Tension
(Figure 4b here)

Figure 4b. Surface tension of PVC with original surface and with the addition of the seven disinfectants. 3 readings of each sample (n=3) was taken with water, formamide and diiodomethane.
Where
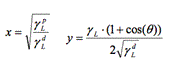
|
Original |
Bioxy H1 |
Bioxy H5 |
Bioxy +1 |
Bioxy +5 |
SH |
HP |
QA |
SV (mN/m) |
24.9 |
13.49 |
14.39 |
29.89 |
26.07 |
23.77 |
23.24 |
24.9 |
Stainless Steel
All the different surfaces of stainless steel are hydrophilic, with a range of 13.5° to 87.1°. The t-tests showed that both Bioxy H5 (13.5°) and Bioxy H1 (15.6°) were significantly lower than the other surfaces.
Wettability
(Figure 5a here)
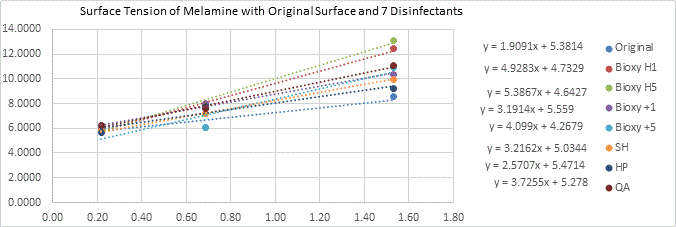
Figure 5a. Wettability (contact angle) of stainless steel with original surface and with the addition of seven disinfectants. 3 readings of each sample was taken (n=3).
Surface Tension
(Figure 5b here)
Figure 5b. Surface tension of stainless steel with original surface and with the addition of the seven disinfectants. 3 readings of each sample(n=3) was taken with water, formamide and diiodomethane.
Where
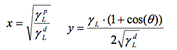
|
Original |
Bioxy H1 |
Bioxy H5 |
Bioxy +1 |
Bioxy +5 |
SH |
HP |
QA |
SV (mN/m) |
35,47 |
69,63 |
70,77 |
22,97 |
36,23 |
29,15 |
35,78 |
30,68 |
Galvanized Steel
There seems to be a wider range for the surfaces of galvanized steel in terms of wettability. Bioxy +1 and sodium hypochlorite are the only ones that are slightly hydrophobic (91.3° and 95.1°, respectively). The t-test showed that only Bioxy H5 was significantly lower than the other surfaces, making it the most hydrophilic.
Wettability
(Figure 6a here)
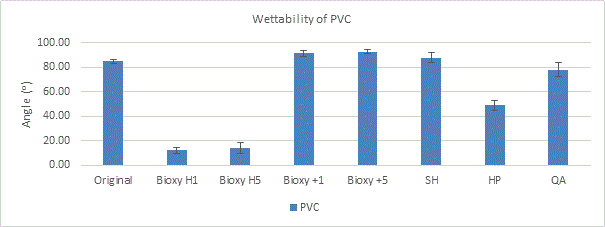
Figure 6a. Wettability (contact angle) of galvanized steel with original surface and with the addition of seven disinfectants. 3 readings of each sample was taken (n=3).
Surface Tension
(Figure 6b here)
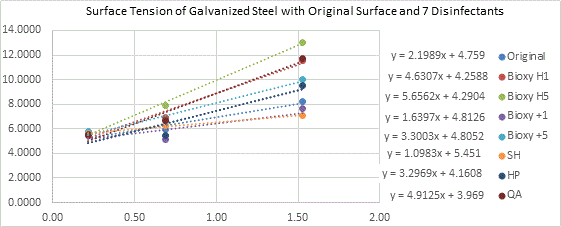
Figure 6b. Surface tension of galvanized steel with original surface and with the addition of the seven disinfectants. 3 readings of each sample(n=3) was taken with water, formamide and diiodomethane.
Where
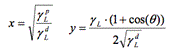
|
Original |
Bioxy H1 |
Bioxy H5 |
Bioxy +1 |
Bioxy +5 |
SH |
HP |
QA |
SV (mN/m) |
27,48 |
39,58 |
50,40 |
25,85 |
33,98 |
30,92 |
28,18 |
39,89 |
Aluminum
The original surface of aluminum and the aluminum surface with hydrogen peroxide are the only ones that are hydrophobic, with angles of 95.4° and 100° respectively. Interestingly, the t-tests showed this time that Bioxy H5 and Bioxy +1 were the ones that were significantly lower than the others, making them the most hydrophilic.
Wettability
(Figure 7a here)
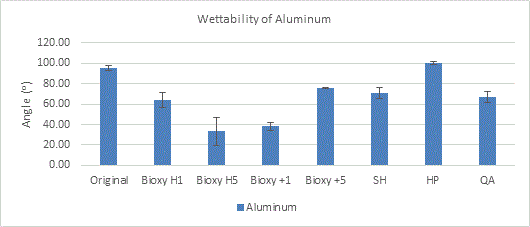
Figure 7a. Wettability (contact angle) of aluminum with original surface and with the addition of seven disinfectants. 3 readings of each sample was taken (n=3).
Surface Tension
(Figure 7b here)
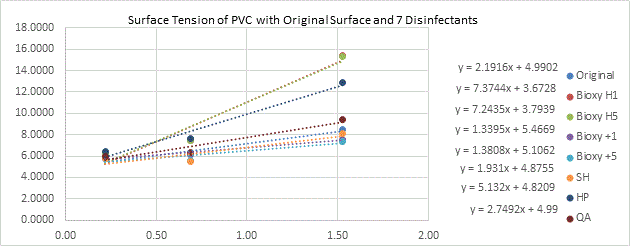
Figure 7b. Surface tension of aluminum with original surface and with the addition of the seven disinfectants. 3 readings of each sample(n=3) was taken with water, formamide and diiodomethane.
Where
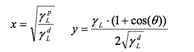
|
Original |
Bioxy H1 |
Bioxy H5 |
Bioxy +1 |
Bioxy +5 |
SH |
HP |
QA |
SV (mN/m) |
38,68 |
39,34 |
59,78 |
56,69 |
33,68 |
35,74 |
36,16 |
40,13 |
The results show a correlation between wettability and surface tension. As the wettability increases (contact angle decreases), the surface tension at the solid-vapor interface generally increases as well. When surface tension at the solid-vapor interface is high, there is a higher affinity for adhesion [16]; hence the water particles will dissipate more throughout the surface, which will cause a lower contact angle. Bioxy H5 is statistically significantly different than all of the other disinfectants except for Bioxy H1 or Bioxy +1 in some cases. This result would entail that Bioxy H5 spreads out the most on surfaces, a valuable characteristic for a disinfectant, as it can disinfect a greater surface area.
According to a study, disinfectants may cause an alteration in surface tension and wettability [20]. When the materials, both polymers and metals, were subjected to disinfectant procedures, there was sometimes a decrease in the contact angle values, especially when Bioxy H5 was used. Therefore, Bioxy H5 increase the values of surface free energy (i.e surface tension) at the solid-vapor interface and decreases the contact angle in relation to the disinfectants, thus allowing a better wettability. Bioxy H5, Bioxy H1 and in some cases Bioxy +1 interfere positively with the adhesion mechanism since they have a high surface tension and wettability on the surfaces. In terms of hydrogen peroxide and sodium hypochlorite, we can observe a higher contact angle and lower surface tension, which would imply its weakness as disinfectant agents. Currently on the market there are different versions of hydrogen peroxide and sodium hypochlorite, which include tension-active agents, to allow for a greater surface tension wettability capability. Furthermore, tension-active agents allow the disinfectant to properly penetrate the microbial structure, which aids in their elimination.
With regards to our previous study, we suspect a correlation with the FTIR results and the ones found in this study. The surfaces with a greater surface tension generally have a higher hydroxyl group peak [21]. This would suggest that the water particles adhere to the surface due to high surface tension, and hence why the hydroxyl group would be detected by the FTIR.
Further analysis needs to be done on whether the increase in wettability affects the surface composition with the increase in humidity (due to possible disinfectant being absorbed by the surface). No other work in the literature has evaluated the effect of these disinfection substances on the wettability and surface tension of these hospital materials.
All the authors thank Natural Science and Engineering Research Council of Canada (NSERC) and Atomes FD for their support.
- Horan TC, Gaynes RP (2004) Surveillance of nosocomial infections. In: Hospital epidemiology and infection control 3rd (Eds.,) Mayhall CG. Philadelphia Lippincortt. Williams and Wilkins pp: 1659-1702
- Madani, Naoufel (2015) Health-Care Associated Infections Rates, Length of Stay and Bacterial Resistance in an Intensive Care Unit of Morocco: Findings of the International Nosocomial Infection Control Consortium (INICC). International Archives of Medicine 2: 29.
- Mireles LK, Sacher E, Yahia LH, Laurent S, Stanicki D (2016) A comparative physicochemical, morphological and magnetic study of silane-functionalized superparamagnetic iron oxide nanoparticles prepared by alkaline coprecipitation. The International Journal of Biochemistry & Cell Biology.
- Drake DR, Paul J, Keller JC (1999) Primary bacterial colonization of implant surfaces. Int J Oral Maxillofac Implants 14: 226-232. [Crossref]
- Quirynen M, Bollen CM (1995) The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man. A review of the literature. J Clin Periodontol 22: 1-14. [Crossref]
- Liu Y1, Zhao Q (2005) Influence of surface energy of modified surfaces on bacterial adhesion. Biophys Chem 117: 39-45. [Crossref]
- Massicotte R (2011) Comparative Study from a Chemical Perspective of Two- and Three-step Disinfection Techniques to Control Clostridium Difficile Spores. Int J Infect Control 7: 1-4.
- Dagher, Fadi, Dori Dagher (2013) Powdered Composition for the Generation of Peracetic Acid and Use Thereof to Sanitize Surfaces. Patent CA.
- Bioxy Afd Inc (2008) Powdered Composition For The Generation Of Peracetic Acid And Use Thereof To Sanitize Surfaces.
- Gittens RA, Scheideler L, Rupp F, Hyzy SL, Geis-Gerstorfer J, et al. (2014) A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater 10: 2907-2918. [Crossref]
- Eriksson C (2004) Implantation of hydrophilic and hydrophobic titanium discs in rat tibia: cellular reactions on the surfaces during the first 3 weeks in bone. Biomaterials 25: 4759-4766.
- Bornstein MM (2008) Bone apposition around two different sandblasted and acid-etched titanium implant surfaces: a histomorphometric study in canine mandibles. Clin Oral Implants Res 19: 233-241
- Zenkiewicz M (2007) Methods for the calculation of surface free energy of solids. Journal of Achievements in Materials and Manufacturing Engineering 24: 137-145.
- Doyle RJ (2000) Contribution of the hydrophobic effect to microbial infection. Microbes Infect 2: 391-400. [Crossref]
- Oliveira R, Azeredo J, Teixeira P, Fonseca AP (2001) The role of hydrophobicity in? bacterial adhesion. In: Gilbert P, Allison D, Brading M, Verran J, Walker J, (Eds.,) Biofilm community interactions: chance or necessity? Cardiff: Bioline. Pp:11-22.
- Prado M, de Assis DF, Gomes BP, Simão RA (2011) Effect of disinfectant solutions on the surface free energy and wettability of filling material. J Endod 37: 980-982. [Crossref]
- Rupp, Frank (2016) A Review on the Wettability of Dental Implant Surfaces: Theoretical and Experimental Aspects. Acta Biomaterialia 10: 2894-2906.
- Liu Y, Zhao Q (2005) Influence of surface energy of modified surfaces on bacterial adhesion. Biophys Chem 117: 39-45. [Crossref]
- Owens DK, Wendt RC (1969) Estimation of the Surface Free Energy of Polymers. J Applied Polymer Science 13: 1741-1747.
- Lad PP, Gurjar M, Gunda S, Gurjar V, Rao NK (2015) The Effect of Disinfectants and a Surface Wetting Agent on the Wettability of Elastomeric Impression Materials: An In Vitro Study. J Int Oral Health 7: 80-83. [Crossref]
- Mireles LK (2016) Interactions of active compounds of disinfectants on metallic and polymeric hospital surfaces.