Abstract
Hepatitis C virus (HCV) infection is a disease with a significant worldwide impact. In Europe and the United States, chronic hepatitis C is the most common cause of chronic hepatic disease and the main indication for liver transplantation. Recurrent hepatitis C infection is universal among transplant recipients who have detectable viremia at the time of transplantation. Hepatitis C treatment was revolutionized with the introduction of safe, powerful direct action antivirals (DAA), which allow the use of multidrug combinations that can selectively inhibit the targets required for viral replication. One of these regimens combined paritarpevir [NS3/4A protease inhibitor], ombitasvir [NS5A inhibitor] and dasabuvir [NS5B polymerase inhibitor], plus ribavirin and was found to be highly effective (SVR rates of 97% in genotype 1). We report the results of a real-world clinical practice study in a single clinical unit in 22 liver graft recipients, transplanted due to cirrhosis caused by genotype 1 HCV with post-transplantation viral recurrence, who received ombitsavir combined with paritaprevir-ritonavir plus dasabuvir and ribavirin.
We found an SVR rate at 12 weeks post-treatment of 100% and a remarkably low rate of adverse events.
Conclusion: oral ombitasvir combined with ritonavir-paritaprevir plus dasabuvir and ribavirin for 24 weeks is a highly effective treatment for eliminating HCV in liver transplant recipients with genotype 1 and scant fibrosis, producing few serious adverse effects.
Keywords
hepatitis C virus; infection; liver transplantation; surgery
Abbreviations
CBC: Complete Blood Count; CMP: Comprehensive Metabolic Panel; DAA: Direct Action Antivirals; DSV: Dasabuvir; HCV: Hepatitis C Virus; OBV: Ombitasvir; PTV: Paritaprevir; PEG-IFN: Pegylated Interferon; r: Ritonavir; RBV: Ribavirin; SVR: Sustained Virologic Response
Introduction
Hepatitis C virus infection is a disease with a significant worldwide impact. According to the World Health Organization, around 130-150 million people (2%-2.5% of the world population) have chronic HCV infection. The recent LANCET analysis of the Global Burden of Disease study estimated about 700.000 annual deaths due to HCV. An estimated 2 to 5 million individuals in Europe are HCV-positive. In Europe and the United States, chronic hepatitis C is the most common cause of chronic hepatic disease and the main indication for liver transplantation.
Recurrent HCV infection is universal among transplant recipients who have detectable viremia at the time of transplantation. The impact of HCV infection on graft histology varies widely, and liver injury can range from mild involvement, even in the presence of a high viral load, to cirrhosis. Some studies suggest that between 20% and 30% of patients who receive a liver transplant due to HCV develop cirrhosis within 5-10 years [1]. After a diagnosis of cirrhosis, the risk of decompensated disease accelerates (17% and 42% at 6 and 12 months, respectively) [2], and patient survival falls significantly (66% and 30% at 1 and 5 years, respectively) [3]. Elimination of the virus after transplantation can reduce the risk of HCV-related complications, such as progression to cirrhosis or graft loss [4,5].
Until recently, the standard of care for treating post-transplantation HCV relapse was pegylated interferon (PEG-IFN) and ribavirin (RBV), with sustained virologic response (SVR) rates of 20%-30% [6], lower than those observed in non-transplanted patients. These low response rates were due, in part, to treatment-limiting side effects. Interferon-based treatments can also induce immunological damage in the liver graft, reducing its survival [7].
Hepatitis C treatment was revolutionized with the introduction of safe, powerful direct action antivirals (DAA), which allow the use of multidrug combinations that can selectively inhibit the targets required for viral replication. Results on the efficacy and safety of these treatments in clinical practice are now available.
Initially reported data on the combination of sofosbuvir and RBV showed a SVR rate of 70% [8]. These results were surpassed by other more effective combinations such as sofosbuvir and simprevir [9] or sofusbuvir and daclatasvir [10].
One of these combinations is ombitasvir with paritaprevir plus dasabuvir. Ombitasvir (OBV) is a NS5A inhibitor formulated in combination with the NS3/4A protease inhibitor paritaprevir (PTV) and the pharmacokinetic potentiator ritonavir (r) which increases peak and trough exposure to the drugs, meaning that PTV can be administered only once a day [11]. This multi-target 3 DAA regimen, administered concomitantly with the non-nucleoside polymerase inhibitor NS5B dasabuvir (DSV), with or without RBV, has shown high rates of SVR in several studies in patients with genotype 1 HCV [12-16].
The Coral-I multicenter, phase III study found this therapy to be both safe and effective in liver transplant recipients, with SVR rates in genotype 1 of 97%, 12 weeks after completing treatment [17].
We report the results of a real-world clinical practice study in a single clinical unit in 22 liver graft recipients, transplanted due to cirrhosis caused by genotype 1 HCV with post-transplantation viral recurrence, who received ombitsavir combined with paritaprevir-ritonavir plus dasabuvir and ribavirin.
Methods
This was an observational, real-world clinical practice, descriptive, longitudinal study with prospective follow-up, performed in the Complexo Hospitalario Universitario A Coruña (north-west Spain). Patients began treatment between March 2015 and August 2015, and were followed up during treatment and for 12 weeks after completion.
Patients receiving liver transplants due to genotype 1 HCV cirrhosis, with post-transplantation viral relapse, aged > 18 years and HCV RNA > 10,000 IU/ml were included. Study patients did not have advanced fibrosis (elastography < 9.4 kPa or liver biopsy with Metavir index ≤2), and had stable levels of immunosuppressive drugs (cyclosporin or tacrolimus). None of the patient presented co-infection with either human immunodeficiency virus (HIV) or hepatitis B virus. All patients gave informed consent in writing before starting treatment.
Patients began treatment with the following regimen: ombitasvir-paritaprevir/ritonavir (1 daily dose of 25 mg ombitasvir, 150 mg paritaprevir and 100 mg ritonavir), dasabuvir (250 mg twice daily) and ribavirin. The ribavirin dose varied depending on hematology and renal function tests. Planned treatment duration was 24 weeks.
On the basis of a pharmacokinetic study of the interaction between these drugs and tacrolimus or cyclosporin, and the recommendations of the Coral-I study [17], the dose of immunosuppressive agents was adjusted as follows: for cyclosporin, one fifth of the total previous dose was administered once a day; for tacrolimus, 0.5 mg was given once a week. Tacrolimus and cyclosporin levels were monitored 1 week after beginning antiviral treatment and after dose adjustments. Immunosuppressive drug doses were modified on the basis of drug levels in blood (trough levels for tacrolimus and C2 levels for cyclosporin).
A total of 5 follow-up visits were performed to adjust antiviral drugs and immunussuppresion: 3 of them were face-to-face visits (at week 4, 12 and end of treatment) and two telephone visits at day 7 after treatment prescription and day 7 after end of treatment. Laboratory tests during treatment were performed in all visits [comprehensive metabolic panel (CMP), complete blood count (CBC), immunosuppressive drug levels]; HCV RNA was measured (TaqMan® HCV Quantitative Test (Roche); detection limit 15UI/ml) at week 4, 12 and end of treatment visits. If RNA was detectable at week 4, it was repeated at week 6. When antiviral treatment was completed, the immunosuppressive drugs were readjusted to the dose administered before starting antiviral treatment.
Follow-up clinical laboratory tests were performed at week 4 after completing treatment (CMP, CBC, immunosuppressive drug levels, and HCV RNA) and at week 12 after completing treatment (CMP, CBC, immunosuppressive drug levels, and HCV RNA).
Statistical analysis
A descriptive analysis was made of all variables collected during the study. Qualitative variables are shown as frequencies and percentages. Quantitative variables are shown as median and range. Efficacy and the rate of adverse effects during the study were analyzed, along with 95% confidence levels. The statistical analysis was performed using SPSS 19.0 for Windows.
Results
A total of 22 patients with genotype 1 (81.8% of patients had genotype 1b and 18.2% had genotype 1a) were included, 77.4 months (mean) after transplantation, of which 81.8% were men. With regard to fibrosis staging, 68.2% had grade 1 fibrosis and 31.8 % had grade 2 fibrosis. Fifty percent (11 patients) had been treated before transplantation (9 with PEG+RBV and 2 with interferon). Tacrolimus was the main immunosuppressive agent for 59.1%, while 27.3% were receiving cyclosporin, and 13.6% single-agent mycophenolate mofetil (MMF) (Table 1).
Table 1. General data.
|
Value |
Sex, men, n (%) |
18 (81.8%) |
Age, years: median (min-max) |
65.5 (51-77) |
HCV genotype
1b
1a |
18 (81.8%)
4 (18.2%) |
Fibrosis n (%)
1
2 |
15 (68.2%)
7 (31.8%) |
Elastography kPa: median (min-max) |
7 (4-7.9) |
Months since transplant: median (min-max) |
77.4 (4.2-215) |
Previous treatment with PEG+RBV/IFN n (%) |
11 (50%) |
HCV RNA IU/ml (min-max) |
1984396 (33000-33640504) |
Main immunosuppressive agent n (%)
Tacrolimus
Cyclosporin
MMF |
13 (59.1%)
6 (27.3%)
3 (13.6%) |
Liver enzymes: median (min-max)
AST IU/L
ALT IU/L
GGT IU/L |
42.5 (20-407)
62.0 (21-496)
78.5 (14-1118) |
Alkaline phosphatase IU/L |
207 (103-598) |
Albumin g/dl |
4.1 (3.1-4.8) |
Baseline glomerular filtration rate in ml/min
n (%)
> 60
30-60
< 30 |
12 (54.5%)
9 (40.9%)
1 (4.5%) |
Baseline creatinine mg/dl median (min-max) |
1.2 (0.9-2.3) |
Baseline total bilirubin mg/dl median (min-max) |
1.0 (0.4-2.7) |
Baseline hemoglobin g/dl median (min-max) |
14.6 (8.7-17.1) |
Tacrolimus and cyclosporin dose adjustments during treatment were performed according to trough levels for tacrolimus and C2 levels for cyclosporin. Two patients receiving tacrolimus and 1 receiving cyclosporin required dose adjustment 1 week after starting treatment. Levels remained very stable in general, and no more dose adjustments were required until treatment completion (Figures 1 and 2).
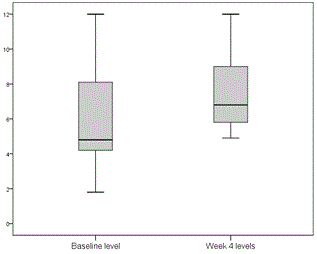
Figure 1. Minimum tacrolimus levels before starting treatment and at week 4 after starting treatment with 3D (ng/ml).
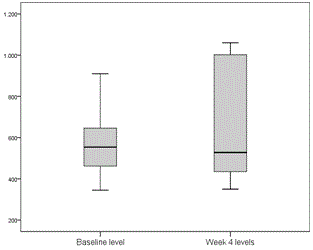
Figure 2. Cyclosporin C2 levels before starting treatment and at week 4 after starting treatment with 3D (ng/ml).
After discontinuation of antiviral treatment, the cyclosporine dose was identical to the pre-treatment dose. In patients receiving tacrolimus, a dose incresase after the end of antiviral treatment was required in four patients.
Efficacy
2021 Copyright OAT. All rights reserv
At week 4 of treatment, 7 patients (31.8%) had detectable HCV RNA. All of them had undetectable HCV RNA at week 6 of treatment. All patients in the study (100%) had undetectable RNA at week 12. One patient discontinued treatment in week 15 (RNA-undetectable at that time) due to poor tolerance (extreme fatigue). The 21 patients who completed the 24 weeks of treatment remained RNA-undetectable until the end of treatment. After completing treatment, RNA at week 4 and week 12 post-treatment was undetectable for all patients (Table 2).
Table 2. Response during and after treatment.
|
HCV <15 IU/ml |
|
n (%) |
95% CI |
During treatment
Week 4
Week 6
Week 12
Week 24 |
15 (68.2%)
22 (100%)
22 (100%)
21* (100%) |
45.1%-86.1%
84.6%-100.0%
84.6%-100.0%
83.9%-100.0% |
After completion of treatment
Week 4
Week 12 |
22 (100%)
22 (100%) |
84.6%-100.0%
84.6%-100.0% |
SVR |
22 (100%) |
84.6%-100.0% |
Treatment failures |
0 (0%) |
0%-15.4% |
*1 patient discontinued treatment in week 15 but was RNA negative
Adverse effects
None of the patients experienced rejection during or after completion of treatment. Adverse effects were observed in 81.8% of patients (5 had more than 1 adverse effect). Ten patients (45.5%) had hyperbilirubinemia, 10 (45.5%) had anemia, 3 (13.6%) headache, 3 (13.6%) nausea, and 1 patient had extreme fatigue requiring treatment discontinuation. Five patients (22.9%) had grade 3 biochemistry abnormalities (2 of them had 2 abnormalities): 3 were hyperbilirubinemia (total bilirubin > 4 mg/dl); and 4 were hemoglobin < 8 g/dl (all 4 patients received a blood transfusion). Treatment did not have to be discontinued due to hyperbilirubinemia. Anemia was believed to be secondary to RBV treatment. The initial dose of RBV ranged between 200 and 1000 mg (90% received 800-1000 mg). Ten patients (45.5%) required ribavirin dose reduction, and 4 of these required ribavirin suspension (the 4 patients with hemoglobin < 8 g/dl).
Kidney function remained stable throughout treatment. No patients presented renal impairment (Table 3: Adverse effects).
Table 3. Adverse effects.
|
n (%) |
95% CI |
Any adverse effect (%) |
18 (81.8) |
59.7%-94.8% |
Treatment discontinuation: n (%) |
1 (4.5%) |
0.1%-22.8% |
Serious adverse effect |
5 (22.9%) |
13.9%-54.9% |
Common adverse effects: n (%)
Hyperbilirubinemia
Anemia
Headache
Nausea
Fatigue |
10 (45.5%)
10 (45.5%)
3 (13.6%)
3 (13.6%)
1 (4.5%) |
22.4%-68.5%
22.4%-68.5%
2.9%-34.9%
2.9%-34.9%
0.1%-22.8% |
Grade 3 biochemical/hematological alterations:
Total bilirubin > 4 mg/dl
Anemia: Hemoglobin < 8 g/dl |
3 (13.6%)
4 (18%) |
2.9%-34.9%
5.2%-40.3% |
Discussion
There is ample evidence from numerous previous studies that survival rates for patients and grafts are significantly lower in patients undergoing liver transplant due to HCV. In this special population, conventional treatments, based on the administration of PEG+RBV, provide sustained virologic response (SVR) rates of 20%-30% [6] along with a significant number of side effects. For this reason, HCV-positive transplant patients have always been considered a difficult-to-treat population with a pressing need for new therapeutic options. The introduction of first-generation protease inhibitors (boceprevir/telaprevir) combined with PEG+RBV did not solve this problem: despite the moderate increase in SRV rates (20%-71%), the high rate of adverse effects, some of which were serious, required early discontinuation of treatment [18-20].
This dilemma was resolved by the development of new direct action antivirals (DAAs), which have truly revolutionized hepatitis C treatment. Indeed, data from both clinical trials and clinical practice have shown SRV rates higher than 90%-95%, in addition to a very low incidence of significant adverse effects.
One of the unquestionable advantages of DAA treatment without interferon is that it can be used in different populations, including those previously categorized as difficult to treat. These include HIV-infected individuals, patients with decompensated cirrhotic disease, and in particular, immunosuppressed transplant recipients. Several studies have been published recently on sofosbuvir-based regimens combined with other DAAs in transplant recipients with genotype 1 infection. SVR rates at 12 weeks post-treatment were consistently higher than 85% (9,10).
In one recent study, Coral-1, a different, non-sofosbuvir-based regimen (combined paritarpevir [NS3/4A protease inhibitor], ombitasvir [NS5A inhibitor] and dasabuvir [NS5B polymerase inhibitor], plus ribavirin) was used, and was found to be highly effective (SVR rates of 97% in genotype 1) [17].
In our study, we reproduced this treatment schedule and were able to confirm its high level of efficacy. We found an SVR rate at 12 weeks post-treatment of 100% and a remarkably low rate of adverse events. One patient did discontinue treatment early (at treatment week 15) due to extreme fatigue, although he too had achieved SVR. This was a patient with significant renal impairment (GFR 23 ml/min) whose fatigue continued despite suspending treatment. It seems possible, then, that the reason for his limited clinical status lay in his comorbidities.
One of the major disadvantages of using the PTV/r/OBV/DSV combination in transplant recipients is the potential for pharmacological interactions, specifically with anti-calcineurins (cyclosporin and tacrolimus), the mainstays of post-transplantation immunosuppression. All our patients required initial dosage readjustment of both drugs, but after that, few modifications were made, and levels remained very stable throughout the 24 weeks of treatment (Figures 1 and 2). None of the patients experienced rejection during or after completion of treatment. The incidence of serious adverse effects is far lower than that reported for regimens containing interferon, and most were attributed to RBV. Specifically, 10 patients (45.5%) required an RBV dose reduction, of which 4 had to discontinue, although this had no effect on SVR. At the time this study was conducted, available evidence with DDA regimens in liver transplant population was scarce and these patients were considered a difficult to treat population, so ribavirin use was widespread. Ribavirin use is not considered necessary in most patients with current data.
Our study has certain limitations, first among them being the small sample size. This, however, is a single-center study with a treatment protocol and well-established follow-up schedule, and all patients were managed in the same way. Secondly, our cohort did not present very advanced liver disease, defined as fibrosis stage ≤ 2, so our results cannot be extrapolated to patients with advanced fibrosis. Finally, treatment duration was set at 24 weeks, following the recommendations of the Summary of Product Characteristics for the European Union in liver transplant recipients. For our patients, who had scant fibrosis, a 12-week treatment period may have been equally effective, despite their immunosuppressive status.
Conclusion
To conclude, oral ombitasvir combined with ritonavir-paritaprevir plus dasabuvir and ribavirin for 24 weeks is a highly effective treatment for eliminating HCV in liver transplant recipients with genotype 1 and scant fibrosis, producing few serious adverse effects. Interactions with immunosuppressants are easy to handle and do not constitute a limitation on the use of this drugs in liver transplant setting.
More evidence is needed to establish the exact duration of treatment and the need to use ribavirin in this population.
Conflicts of interests
The authors of the present manuscript do not have any conflict of interest to disclose.
Financial support
This is a real-world clinical practice study without financial support.
References
- Berenguer M, Ferrell L, Watson J, Prieto M, Kim M, et al. (2000) HCV-related fibrosis progression following liver transplantation: increase in recent years. J Hepatol 32: 674-684. [Crossref]
- Berenguer M, Prieto M, Rayón JM, Mora J, Pastor M, et al. (2000) Natural history of clinically compensated HCV-related graft cirrosis after liver transplantation. Hepatology 32: 852-858. [Crossref]
- Saab S, Niho H, Comulada S, Hiatt J, Durazo F, et al. (2005) Mortality predictors in liver transplant recipients with recurrent hepatitis C cirrhosis. Liver Int 25: 940-945. [Crossref]
- Terrault NA, Berenguer M (2006) Treating hepatitis C infection in liver transplant recipients. Liver Transpl 12: 1192-1204. [Crossref]
- Crespo G, Mariño Z, Navasa M, Forns X (2012) Viral hepatitis in liver transplantation. Gastroenterology 142: 1373-1383. [Crossref]
- Gordon FD, Kwo P, Vargas HE (2009) Treatment of hepatitis C in liver transplant recipients. Liver Transpl 15: 126-135. [Crossref]
- Levitsky J, Fiel MI, Norwell JP, Wang E, Watt KD, et al. (2012) Risk for immune mediated graft dysfunction in liver transplant recipients with recurrent HCV infection treated with pegylated interferon. Gastroenterology 142: 1132-1139. [Crossref]
- Charlton M, Gane E, Manns MP, Brown RS Jr, Curry MP, et al. (2015) Sofosbuvir and ribavirin for treatment of compensated recurrent hepatitis C virus infection after liver transplantation. Gastroenterology 148: 108-117. [Crossref]
- Pungpapong S, Aqel B, Leise M, Werner KT, Murphy JL, et al. (2015) Multicenter experience using simeprevir and sofosbuvir with or without ribavirin to treat hepatitis C genotype 1 after liver transplant. Hepatology 61: 1880-1886. [Crossref]
- Poordad F, Schiff ER, Vierling JM, Landis C, Fontana RJ, et al. (2016) Daclatasvir with sofosbuvir and ribavirin for hepatitis C virus infection with advanced cirrhosis or post-liver transplantation recurrence. Hepatology 63: 1493-1505. [Crossref]
- Menon RM, Klein CE, Lawal AA (2009) Phamacokinetics and tolerability of the HCV proteasa inhibitor ABT-450 following single ascending doses in healthy adult volunteers with and without ritonavir. Global Antiviral 5: 53.
- Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, et al. (2014) ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med 370: 1973-1982. [Crossref]
- Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson DR, et al. (2014) Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med 370: 1594-1603. [Crossref]
- Zeuzem S, Jacobson IM, Baykal T, Marinho RT, Poordad F, et al. (2014) Retreatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med 370: 1604-1614. [Crossref]
- Andreone P, Colombo MG, Enejosa JV, Koksal I, Ferenci P, et al. (2014) ABT-450, ritonavir, ombitasvir, and dasabuvir achieves 97% and 100% sustained virologic response with or without ribavirin in treatment-experienced patients with HCV genotype 1b infection. Gastroenterology 147: 359-365. [Crossref]
- Ferenci P, Bernstein D, Lalezari J, Cohen D, Luo Y, et al. (2014) ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med 370: 1983-1992. [Crossref]
- Kwo PY, Mantry PS, Coakley E, Te HS, Vargas HE, et al. (2014) An interferon-free antiviral regimen for HCV after liver transplantation. N Engl J Med 371: 2375-2382. [Crossref]
- Pungpapong S, Aqel BA, Koning L, Murphy JL, Henry TM, et al. (2013) Multicenter experience using telaprevir or boceprevir with peginterferon and ribavirin to treat hepatitis C genotype 1 after liver transplantation. Liver Transpl 19: 690-700. [Crossref]
- Coilly A, Roche B, Dumortier J, Leroy V, Botta-Fridlund D, et al. (2014) Safety and efficacy of protease inhibitors to treat hepatitis C after liver transplantation: a multicenter experience. J Hepatol 60: 78-86. [Crossref]
- Verna EC, Saxena V, Burton JR Jr, O'Leary JG, Dodge JL, et al. (2015) Telaprevir- and Boceprevir-based Triple Therapy for Hepatitis C in Liver Transplant Recipients With Advanced Recurrent Disease: A Multicenter Study. Transplantation 99: 1644-1651. [Crossref]


