Older adults are more likely to be required to face the problems of deteriorating movement control due to ageing and poor visuomotor adaptation is believed to be one of the important contributors to the problems. Therefore, we assessed motor performance together with gaze behaviors in young and older adults when they were performing computer-based reaching tasks aiming to examine the potential impact of ageing on visuomotor behaviors and adaptation. In this study, visuomotor behaviors in computer-based reaching tasks were quantitatively evaluated under providing online visual feedback or blocking online visual feedback (simulated visual deficiency) conditions. Results revealed that ageing affects motor performance of the reaching tasks significantly in both visual feedback conditions. Older adults performed distinctive gaze behaviors when compared with the young adults. It implies that simulated visual deficiency in the blocking online visual feedback condition may work as a stimulus to cause extra perceptive load during movement execution and, more importantly, ageing induces slower visuomotor adaptation. Therefore, visual deterioration may slow down the process of visuomotor adaptation. Consequently, the results of present study provide us with new insights in how to further improve the Geriatric rehabilitative training methods for older adults in the context of augmenting visuomotor behaviors, for example, by utilizing a well-designed errorless training methodology to enhance movement automaticity and visuomotor adaption during motor rehabilitation in Geriatric population.
ageing, Gaze behaviour, visuomotor behaviours, reaching, rehabilitation
Older adults are more prone to suffer from motor control problems due to ageing. Previous studies indicated that ageing-related deterioration undermines daily life activities of older adults in different aspects. Older adults are more vulnerable than young adults with decreased visuospatial working memory [1,2], deteriorated cognitive processes [3,4], increased movement variability and instability [5], decreased directional sensitivity of balance [6] and decreased muscle strength [7].
Ageing creates differences in neural control of movement within different brain regions, such as the prefrontal cortex and basal ganglia, which in turn affects motor performance [8]. Motor control and learning is a manifold integration of cognitive and sensorimotor systems [9]. Older adults may confront some problems due to motor sensory deficiency [10]. As a result, these ageing-related changes of sensory system may result in movement instability and impreciseness [11], which affect mobility of the older adults. Additionally, due to the relatively poorer efficiency of the musculoskeletal system among older adults, the process of movement execution can become slow and unstable. Therefore, motor control indeed becomes weak among the ageing population in both peripheral and central nervous systems [12]. All these ageing-related changes that occur in older adults may affect their ability in motor control and learning causing impairments of movement speed, movement stability, force control and coordination [13].
In addition, ageing affects motor performance of older adults adversely in daily life activities and ageing-related visual degeneration further exacerbates this problem [14]. For instance, older adults who suffered from ageing-related visual impairment and degeneration, such as presbyopia, may deteriorate their motor performance [15]. Visual impairments such as changes in visual field, acuity or contrast sensitivity are associated with other sensory deficiency that may increase movement instability [16]. Older adults have limited visual field and it may be caused by slow visual processing speed due to ageing [17]. The slow visual processing speed in older adults may affect visual attention as well, which was shown to be associated with mobility problems independently [18]. Ageing-related visual impairments degraded performance in Useful Field of View Test (UFOV). It indicated that older adults would have difficulties to obtain visual information which often manifests as slow cognitive processing speed [19].
Generally, complicated movements can be broken down into its basic movements. Reaching, one of the essential parts of many complicated movements, is one of the best basic movements to study the mechanism of visuomotor adaptation [20]. Various studies reviewed ageing effects on motor performance or gaze behaviors independently, but scanty studies discussed the effects of ageing-related visual degeneration or deterioration concerning both motor performance and gaze behaviors simultaneously when conducting reaching task. Therefore, in order to investigate the potential impact of ageing on visuomotor adaptation, we assessed motor performance and gaze behaviors in young and older age groups with computer-based reaching tasks. Visuomotor behaviors in reaching were quantitatively assessed under providing online visual feedback or blocking online visual feedback (simulated visual deficiency) conditions. We hypothesized that: 1, Ageing deteriorates visuomotor performance in the computer-based reaching task; 2, Simulated visual deficiency induces different strategies of visuomotor adaptation in young and older adults.
participants
Sixty healthy young adults (Mean age=24.49 years, SD=2.12) and thirty-seven older adults (Mean age=70.07 years, SD=2.37) participated in this study. Young adults (N=60) were randomly allocated into either Blocking Online Visual Feedback (BOVF) subgroup (N=30) or Providing Online Visual Feedback (POVF) subgroup (N=30). On the other hand, older adults (N=37) were allocated randomly into the same subgroups, with BOVF (N=22) and POVF (N=15). All participants had normal or corrected to normal vision and met the inclusion criteria of participation. They had no history of retina, cerebral vascular disease, Parkinson’s disease or any other neurological impairment before. This experiment was carried out in accordance with the Human Research Ethics Committee (HREC) in the University of Hong Kong for ethical clearance for research involving human participants and written consents were received before the start of any experimental procedure.
Material and apparatus setup
Right eye’s movement of the participants was recorded by the EyeLink II desktop mount system (SR Research, Ontario, Canada) at a sampling rate of 500 Hz. Stimulus presentation and response recording were controlled by the Experiment Builder (SR Research, Ontario, Canada). The experimental task instructions were displayed full-screen with a resolution of 1024 by 768 pixels on a 23-inch monitor. The distance between monitor and participants was kept at 70 cm from the participants’ eyes. Additionally, participants handled a mouse (Wireless Mouse M185, Logitech Co.) and conducted reaching tasks in the horizontal plane. Each participant independently conducted the reaching task during every single trial.
Procedure
All participants were invited to complete a 20-trial computer-based reaching tasks in the experiment. A trial began with drift calibration fixation in the center on a white background at the monitor. It was followed by a new stimulus array consisting of mouse-controlled cursor “x” in black, start position in the center of the screen with square in white and a target circle in red. The target radius was fixed to 80 pixels. The distance between the start position to center of target circle was fixed to 300 pixels. The target circle appeared at a randomly selected direction in 20 trials. Once participants moved the mouse-controlled cursor “x”, the online trajectory of the cursor would be blocked with Blocking Online Visual Feedback (BOVF) group and only provided with the click position as the end position feedback to participants in this group. However, cursor appeared throughout the trial under Providing Online Visual Feedback (POVF) group. During all reaching tasks, participants were required to control the cursor “x” and moved it to the target center as soon and as accurate as possible. Before formal tests, participants were arranged to complete 20 practice trials to make sure their understanding of the experimental task.
Motor performance
The ageing-related impacts by different visual feedback patterns on the motor performance of hand-controlled mouse cursor in reaching tasks are shown in Table 1 and Figure 1. Motor performance including movement time, distance to the target center and accurate number among 20 trials in the test, were analyzed in a series of 2x2, Group (older adults, young adults) X Visual Feedback Pattern (BOVF, POVF) two-way Analysis of Variance (ANOVA) with repeated measures.
Table 1. Outcome of ANOVA with repeated measures for different motor performance parameters
Movement time |
|
age groups |
F (1, 1841) = 780.456, p<0. 001, η2=0.298 |
visual feedback patterns |
F (1, 1841) = 260.312, p<0. 001, η2=0.124 |
age groups*visual feedback patterns |
F (1, 1841) = 55.041, p<0. 001, η2=0.029 |
Distance to the target center |
age groups |
F (1, 947) = 143.396, p<0. 001, η2=0.069 |
visual feedback patterns |
F (1, 947) = 2415.918, p<. 001, η2=0.554 |
age groups*visual feedback patterns |
F (1, 947) = 116.752, p<. 001, η2=0.057 |
Accurate number |
age groups |
F (1, 97) = 13.929, p<0. 001, η2=0.126 |
visual feedback patterns |
F (1, 97) = 438.579, p<0. 001, η2=0.819 |
age groups*visual feedback patterns |
F (1, 97) = 14.337, p<0. 001, η2=0.129 |
Note: ANOVA = Analysis of Variance
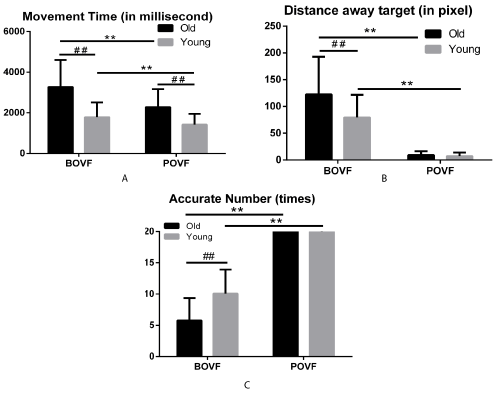
Figure 1. Movement time (A), distance away from the target center(B),accurate number (C) as the motor response on the trials, for old and young adults, in BOVF or POVF. Error bars indicates standard error of the mean. Note: ** indicates significant differences due to visual feedback patterns (p<.001) and ## indicates significant differences due to age groups (p<.001).
Movement time
Older adults took longer movement time to finish the reaching than the young adults under both BOVF (3271.722ms vs. 1786.702ms, p<.001) and POVF (2282.432ms vs. 1420.643ms, p<.001) conditions. The older adults with BOVF cost longer movement time than those with POVF (3271.722ms vs. 2282.432ms, p<.001) and the same effect appeared on the young adults (1786.702ms vs.1420.643ms, p<.001). These results indicated that older adults had longer movement time in reaching than young adults, no matter under BOVF or POVF conditions. However, simulated vision deficits affected reaching motor movement time in older adults to a greater extent than young adults.
Distance to the target center
Older adults with BOVF reached farer distance away from the target center than those older adults with POVF (122.359 vs. 9.295 pixel, p<.001) and the same effect appeared on the young adults (79.402 vs. 7.089 pixel, p<.001). Also, the older adults took a farer distance than the young adults with BOVF (122.359 vs. 79.402 pixel, p<.001) while no difference was found with POVF between the two age groups (9.295 vs. 7.089 pixel, p=.423). The results illustrated that BOVF extended distance to the target center in reaching among older and young adults. BOVF affected older adults more than young adults. However, the effect was not salient under POVF condition.
Accurate number
Older adults had fewer accurate trials reaching to the target than young adults under BOVF (5.762 vs. 10.091, p<.001) condition while no difference was found under POVF condition between the two age groups (20.000 vs.19.969, p=.971). Additionally, older adults with POVF completed the reaching task more accurately than older adults with BOVF (20.000 vs. 5.762, p<.001). The same effect appeared on the young adults (19.969 vs. 10.091, p<.001) as well. These results illustrated that BOVF significantly decreased accurate number within the 20-trial reaching test among older and young adults than POVF condition. BOVF induced more motor errors among older than young adults while this effect was not significant under POVF condition.
Gaze behaviors were analyzed in a series of 2x2, Group (older adults, young adults) X Visual Feedback Pattern (BOVF, POVF) two-way Analysis of Variance (ANOVA) with repeated measures. Various ageing-related differences on gaze behaviors in reaching between older and young adults were found which affected gaze behaviors in different extent by different visual feedback patterns, for example, first fixation duration, first fixation initiation time, dwelling fixation duration in the target, fixation count in the target and run count in the target (Table 2, Figure 2 and 3).
Table 2. Outcome of ANOVA with repeated measures for different gaze behaviors parameters
First fixation duration |
age groups |
F (1, 235) = 96.915, p<0. 001, η2=0.292 |
visual feedback patterns |
F (1, 235) = .032, p=0.857, η2 <0.001 |
age groups*visual feedback patterns |
F (1, 235) = 17.535, p<0. 001, η2=0.069 |
First fixation initiation time |
age groups |
F (1, 236) = 6.887, p =0. 009, η2=0.028 |
visual feedback patterns |
F (1, 236) = 52.234, p <0. 001, η2=0.181 |
age groups*visual feedback patterns |
F (1, 236) = 5.856, p =0. 016, η2=0.024 |
Dwelling fixation duration in the target |
age groups |
F (1, 234) = 27.594, p<0. 001, η2=0.105 |
visual feedback patterns |
F (1, 234) = .776, p=0. 379, η2=0.003 |
age groups*visual feedback patterns |
F (1, 234) = 12.157, p<0. 001, η2=0.049 |
Fixation count in the target |
age groups |
F (1, 231) = 135.716, p<0.001, η2=0.370 |
visual feedback patterns |
F (1, 231) = 26.608, p<0.001, η2=0.103 |
age groups*visual feedback patterns |
F (1, 231) = 1.192, p=0.276, η2=0.005 |
Run count in the target |
age groups |
F (1, 235) = 152.641, p<0. 001, η2=0.394 |
visual feedback patterns |
F (1, 235) = 110.985, p<0. 001, η2=0.321 |
age groups*visual feedback patterns |
F (1, 235) = 21.676, p<0. 001, η2=0.084 |
Note: ANOVA = Analysis of Variance
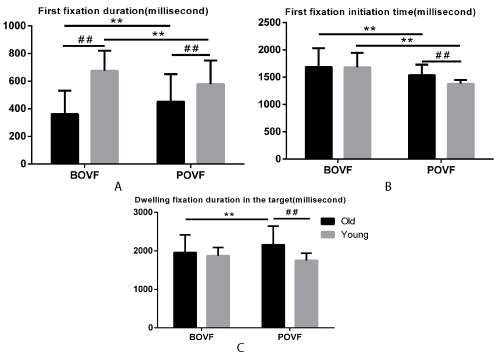
Figure 2. First fixation duration (A), first fixation initiation time (B), dwelling fixation duration (C) for old and young adults, in BOVF or POVF. Error bars indicates standard error of the mean. Note: ** indicates significant differences due to visual feedback patterns (p<.001) and ## indicates significant differences due to age groups (p<.001).
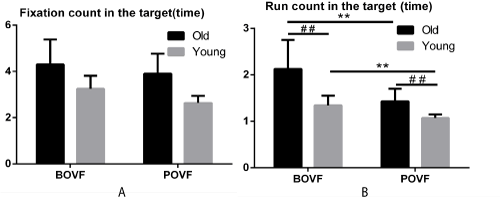
Figure 3. Fixation (A), run count (B) for old and young adults, in BOVF or POVF. Error bars indicates standard error of the mean. Note: ** indicates significant differences due to visual feedback patterns (p<.001) and ## indicates significant differences due to age groups (p<.001).
First fixation duration. First fixation duration is the time (ms) of the first gaze fixation event within the target and long first fixation duration means long time to process the visual information for the movement. Older adults took shorter first fixation duration than the young adults no matter under BOVF (362.296 vs. 675.164ms, p<.001) or POVF (451.640 vs. 577.779ms, p<.001) conditions. Older adults with BOVF costed shorter first fixation duration in the target than those with POVF (362.296 vs. 451.640ms, p<.001) whereas young adults with BOVF took longer first fixation duration than those with POVF (675.164 577.779ms, vs. p<.001). The results suggested that older adults conducted shorter first fixation duration than young adults no matter under BOVF or POVF conditions. However, older adults with BOVF had shorter first fixation duration in the target than those with POVF while this influence reversed among young adults. This result implies that ageing shortened the first fixation duration generally. Simulated visual deficiency affects older adults to a greater extent than young adults in first gaze fixation duration.
First fixation initiation time. First fixation initiation time is the start time of the first fixation that enters the target. Early initiation for the movement is regarded as a quick response for the visual stimuli or better preparation for the following movement. Older adults with BOVF had a delay initiation of first fixation than those with POVF (1686.261 vs 1537.407ms, p=.001) and the same effect appeared in young adults (1679.935 vs.1381.214ms, p<.001). Older adults took a later initiation of a fixation to the target than the young adults under POVF (1537.407 vs. 1381.214ms, p<.001) condition while there was no difference between the two age groups under BOVF (1686.261 vs.1679.935ms, p=.885). The result revealed that POVF condition delay the first fixation initiation time. Besides, the ageing effect was essential only under POVF condition but not significant under BOVF condition.
Dwelling fixation duration in the target. Dwelling fixation duration is the summation of the duration across all fixations in the target and this variable is related to velocity of the cognitive visual processing. Longer dwelling fixation duration means a slower speed to process the visual information provided. Older adults had longer dwelling fixation duration in the target than the young adults under POVF (2162.317 vs. 1752.460ms, p<.001) condition while no difference was found with BOVF between two age groups (1957.498 vs.1874.665ms, p=.215). Older adults with BOVF took a shorter dwelling fixation duration in the target than those with POVF (1957.498 vs. 2162.317ms, p<.001). The results indicated that older adults with POVF had longer dwelling fixation duration in the target than those with BOVF. Moreover, older adults took longer dwelling fixation duration throughout the trial in the target than young adults under POVF condition. These results indicated that ageing affects dwelling fixation duration in the target and older adults might conduct longer duration to process the reaching task than young adults.
Fixation count in the target. Besides, accounting for fixation count (identified as total fixations falling in the target), the interaction between age groups and visual feedback patterns was not significant (F (1, 231) = 1.192, p=.276, η2=.005). The main effect of age group was significant (F (1, 231) = 135.716, p<.001, η2=.370). Also, the main effect of visual feedback pattern was significant (F (1, 231) = 26.608, p<.001, η2=.103). These results suggested that older adults had more fixation count in the target than the young adults under both visual feedback patterns and BOVF condition would result in more fixations in the target than POVF condition among two age groups.
Run count in the target. Run count in the target was identified as how many times participants return the gaze to the target, where they scanned back and forth. Older adults took more run count than the young adults with BOVF (2.126 vs. 1.343, p<.001) condition and the same trends found with POVF (1.427 vs. 1.072, p<.001) condition. Older adults with BOVF conducted more run count in the target than those with POVF (2.126 vs. 1.427, p<.001). The same effect appeared on the young adults (1.343 vs. 1.072, p<.001). No matter under BOVF or POVF condition, older adults created more run count in the target than young adults. BOVF condition induced more run count than POVF among older and young adults. This result suggested that ageing affects consistently under BOVF or POVF condition that augments the run count to the target.
In the present study, it was found that older adults have different responses in motor performance and gaze behaviors under simulated visual deficiency compared to the young adults. Previous studies suggested that visual information facilitates motor planning and online control of movement execution [21] [22], which is essential for the effective motor performance [23]. Visual system may update the online visual information based on the environmental situations during movement execution in order to guide and adjust motor responses promptly [24]. The online visual information contributes to cognitive integration while the movement is executing that can be presented as gaze behaviors (e.g., fixation or saccade) [25]. Therefore, we attempted to analyze gaze behaviors under BOVF or POVF conditions to explore the potential processing of visual information that would be required during movement execution. In addition, kinematic data, such as movement time that measures motor performance, was also recorded. Since gaze behaviors and motor performance could be obtained simultaneously in the present study, this might further examine the relationship between gaze behaviors and motor performance during movement execution with different visual information processes.
The study results suggested that BOVF condition resulted in longer movement time and poorer reaching accuracy in both young and older adults. Additionally, older adults have poorer motor performance and gaze behaviors under BOVF condition compared to the young adults. The degraded motor performance implies that disruption of visual information processing weakens automaticity of movements and visual deficits seem to affect the motor performance significantly more in older adults than young adults.
Concerning the gaze behaviors in reaching, older adults seem to be more vulnerable than young adults under visual deficiency. For the First fixation duration, no matter in BOVF or POVF condition, older adults had significantly shorter first fixation duration than young adults. Previous studies indicated that longer first fixation duration implicated a more effective coordination of gaze and motor systems [26].
People with longer first fixation duration can obtain more information about the reaching target in order to prepare for the motor responses, which leads to more accurate movement execution. However, our results suggested that the motor and gaze coordination may break down when suffering from the visual deficiency. Therefore, longer first fixation duration can be regarded as an indicator to measure whether performers maintain a goal-directed focus [27]. Consequently, the result indicated that older adults may prefer to make less effort and therefore utilize less time to process visual information on the target. The reduced effort in older adults, however, may lead to the insufficient coordination of motor and visual systems. On the other hand, older adults had shorter first fixation duration in BOVF condition than those in POVF condition. On the contrary, young adults had longer first fixation duration in BOVF condition than those in POVF condition. This result indicated that BOVF increases the first fixation duration among young adults. It implies that older adults were affected by visual deficiency more due to ageing and therefore resulting in a weak visual adaptation capability. However, young adults would attempt to take longer first fixation duration under BOVF condition aiming to obtain visual information more smoothly and effectively. The visual adaptation to the visual deficiency among young adults (but not the older adults) would help the young adults to modify and enhance their gaze strategies. Consequently, considering gaze and motor performance together, poor visual adaptation may result in poor motor performance because of the weak coordination of the visual and motor systems. For the First fixation initiation time, it can be identified as the time of participant’s first fixation on the target prior to the initiation of a movement. Under POVF condition, young adults initiated the gaze faster than older adults. Under BOVF condition, older and young adults had similar time to initiate the gaze, which suggested that ageing effect may not saliently work on this variable. Young adults initiated the gaze fixation on target earlier under POVF, which implied the efficiency in orientation of attention [28]. Early and accurate fixation on the target might generate precise movements as well [29]. For the Dwelling fixation time in the target, previous studies indicated that older adults are vulnerable to use online visual feedback rapidly, store or retrieve visuospatial information accurately during movement execution [30,31]. Due to the poor capability in storing or retrieving visuospatial information, older adults would like to make more efforts to prepare and plan their movements. Besides, longer dwelling fixation duration among older adults indicated an extended visual information processing time and might be related to degrade executive function abilities [32] and the slow cognitive processing speed [33]. In this study, the results revealed that older adults conducted a longer dwelling fixation time in the target and more fixation on the target by visually reaching and tracking the environment, possibly in an attempt to reach the target under BOVF more accurately. For the Fixation count in the target, it was found that older adults conducted more fixation counts than young adults and BOVF seduced more fixations than that of POVF. More fixations on the target means more direct attention to the target [34] and implied the higher level of attentional control [35]. It implies that older adults need more attention to the target and the visual deficiency may induce more attentional control during reaching. However, more fixations to the same visual information may result in a lower searching efficiency to potential target because of a poor arrangement of the visual stimuli [36,37]. For the run count in the target, it is defined as the frequency that participants return the gaze to the target when they scanned back and forth [38]. The run count can also be viewed as a measure that represents the perceived difficulty of the task [39]. The higher difficulty to deal with the complex information, the more re-fixated on the specific target area and therefore more run count [40]. In the present study, older adults had more run count than young adults in either BOVF or POVF conditions, ageing may worsen the re-fixation to the target.
The two factors (visual feedback pattern and age group) affect motor performance and gaze behaviors significantly. BOVF may be a stimuli to induce an accumulative perceptive load and then generates deterioration of motor performance [41]. In this study, the simulated visual information deficits (BOVF) may induce visuospatial errors using explicit cognitive strategies. However, when participants were adapted to the visual stimuli, the accumulation of explicit knowledge reduced progressively and therefore the potential problem of excessive perceptive load may be solved. On the other hand, the effects of ageing change visual information acquirement during movements execution process, which correspondingly leads to distinct gaze behaviors [42]. Older adults shrink the area of space to extract useful visual information and this may be partly compensated by the illicit saccades. This can be explained that more fixation would be made in the target in aged group [43]. Accounting for effects of the two factors (visual feedback pattern and age group) simultaneously, older adults may obtain a poor adaptation under BOVF condition because BOVF would break movement automaticity and makes the execution of the movement with more explicit knowledge. However, older adults may have the problem of lower efficiency to utilize explicit knowledge in assisting visuomotor behaviors, resulting in the declines of visuomotor adaptation because of the difficulty to transform visual information [44,45]. Therefore, BOVF might deteriorate visuomotor performance and ageing effects induce less adaptive visuomotor adaptation as well.
In this study, the two visual feedback conditions (i.e., blocking or providing online visual feedback of hand controlled cursor in the computer-based reaching tasks) affect young and older adults differently. Ageing affects motor performance of reaching tasks significantly in both visual feedback conditions and older adults performed distinctive gaze behaviors when compared with the young adults. It suggests that visual deficiency may act as a stimulus to create extra perceptive load during movement execution. Ageing induces slower visuomotor adaptation because visual degeneration and deterioration may act as the causes to decelerate the process of visuomotor adaptation. Indeed, the current study results provide us with novel insights in how to enhance the current Geriatric rehabilitative training methods by utilizing errorless training methodology from rehabilitation psychology literature [46], if follow-up studies can provide additional solid evidence, in which movement automaticity and visuomotor adaptation of older adults are highly likely to be improved after specific errorless training.
This study was supported by the postgraduate research support funding of institute of human performance in the University of Hong Kong. Part of the results in this manuscript was accepted to be presented in the 19th International Conference on Sport Medicine and Sport Science (ICSMSS 2017). The authors would like to express their gratitude to all the participants in this study. The authors are also thankful to Miss Mei Yee Leung, Miss Debbie Chan and Dr. Donghyun Ryu for their assistance in the study.
- Rowe G, Hasher L, Turcotte J (2008) Age differences in visuospatial working memory. Psychol Aging 23: 79-84. [Crossref]
- Rowe G, Hasher L, Turcotte J (2009) Age and synchrony effects in visuospatial working memory. Q J Exp Psychol (Hove) 62: 1873-1880. [Crossref]
- Peretti CS, Danion JM, Gierski F, Grangé D (2002) Cognitive skill learning and aging: a component process analysis. Arch Clin Neuropsychol 17: 445-459. [Crossref]
- Glisky EL (2007) Changes in cognitive function in human aging. Brain aging: Models, methods, and mechanisms. Boca Raton (FL): CRC Press/Taylor & Francis, pp: 3-20. [Crossref]
- Darling WG, Cooke JD, Brown SH (1989) Control of simple arm movements in elderly humans. Neurobiol Aging 10: 149-157. [Crossref]
- Allum JHJ, Carpenter MG, Honegger F, Adkin A L, B R Bloem (2002) Age-dependent variations in the directional sensitivity of balance corrections and compensatory arm movements in man. J Physiol 542: 643-663. [Crossref]
- Dubost V, Beauchet O, Manckoundia P, Herrmann F, Mourey F (2005) Decreased trunk angular displacement during sitting down: an early feature of aging. Phys Ther 85: 404-412. [Crossref]
- Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, et al., (2010) Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev 34: 721-733. [Crossref]
- Georgopoulos AP (2000) Neural aspects of cognitive motor control. Curr Opin Neurobiol 10: 238-241. [Crossref]
- Woollacott MH, Shumway-Cook A, Nashner LM (1986) Aging and posture control: changes in sensory organization and muscular coordination. Int J Aging Hum Dev 23: 97-114. [Crossref]
- HELD R, FREEDMAN SJ (1963) PLASTICITY IN HUMAN SENSORIMOTOR CONTROL. Science 142: 455-462. [Crossref]
- Ketcham CJ, Seidler RD, Van Gemmert AW, Stelmach GE (2002) Age-related kinematic differences as influenced by task difficulty, target size, and movement amplitude. J Gerontol B Psychol Sci Soc Sci 57: 54-64. [Crossref]
- Ketcham, CJ, Stelmach GE (2004) Movement control in the older adult. Hardcopy Version at National Academies Press. [Crossref]
- Owsley C (2011) Aging and vision. Vision Res 51: 1610-1622. [Crossref]
- Kline DW, Kline TJ, Fozard JL, Kosnik W, Schieber F, et al. (1992) Vision, aging, and driving: The problems of older drivers. J Gerontol 47: 27-34. [Crossref]
- Dhital A, Pey T, Stanford MR (2010) Visual loss and falls: a review. Eye 24: 1437-1446. [Crossref]
2021 Copyright OAT. All rights reserv
- Ball KK, Beard BL, Roenker DL, Miller RL, Griggs DS (1988) Age and visual search: expanding the useful field of view. J Opt Soc Am A 5: 2210-2219. [Crossref]
- Owsley C, McGwin G (2004) Association between visual attention and mobility in older adults. J Am Geriatr Soc 52: 1901-1906. [Crossref]
- Elliott AF, O'connor ML, Edwards JD (2014) Cognitive speed of processing training in older adults with visual impairments. Ophthalmic Physiol Opt 34: 509-518. [Crossref]
- Thoroughman KA, Shadmehr R (2000) Learning of action through adaptive combination of motor primitives. Nature 407: 742-747. [Crossref]
- Buneo CA, Andersen RA (2006) The posterior parietal cortex: sensorimotor interface for the planning and online control of visually guided movements. Neuropsychologia 44: 2594-2606. [Crossref]
- Khan MA, Franks IM, Elliott D, Lawrence GP, Chua R, et al. (2006) Inferring online and offline processing of visual feedback in target-directed movements from kinematic data. Neurosci Biobehav Rev 30: 1106-1121. [Crossref]
- Harle SK, Vickers JN (2001) Training quiet eye improves accuracy in the basketball free throw. The Sport Psychologist 15: 289-305.
- Land MF (2009) Vision, eye movements, and natural behavior. Vis Neurosci 26: 51-62. [Crossref]
- Uiga L, Cheng KC, Wilson MR, Masters RS, Capio CM (2015) Acquiring visual information for locomotion by older adults: A systematic review. Ageing Res Rev 20: 24-34. [Crossref]
- Vine SJ, Moore LJ, Cooke A, Ring C, Wilson MR (2013) Quiet eye training: A means to implicit motor learning. Int. J. Sport Psychol 44: 367-386.
- Wilson M, Richards H (2011) Putting it together: Skills for pressure performance. Performance psychology :p. 337-360.
- Gervais SJ, Holland AM, Dodd MD (2013) My eyes are up here: The nature of the objectifying gaze toward women. Sex Roles 69: 557-570.
- Moore LJ, Vine SJ, Cooke A, Ring C, Wilson MR (2012) Quiet eye training expedites motor learning and aids performance under heightened anxiety: The roles of response programming and external attention. Psychophysiology 49: 1005-1015. [Crossref]
- Cheng KC, McKay SM King EC, Maki BE (2012) Does aging impair the capacity to use stored visuospatial information or online visual control to guide reach-to-grasp reactions evoked by unpredictable balance perturbation? J Gerontol A Biol Sci Med Sci 67: 1238-1245. [Crossref]
- Cheng KC, Pratt J, Maki BE (2013) Do aging and dual-tasking impair the capacity to store and retrieve visuospatial information needed to guide perturbation-evoked reach-to-grasp reactions? PLoS One 8: p. e79401. [Crossref]
- Lavie N (2005) Distracted and confused?: selective attention under load. Trends Cogn Sci 9: 75-82. [Crossref]
- Di Fabio RP, Zampieri C, Henke J, Olson K, Rickheim D, et al. (2005) Influence of elderly executive cognitive function on attention in the lower visual field during step initiation. Gerontology 51: 94-107. [Crossref]
- Guastella AJ, Carson DS, Dadds MR, Mitchell PB, Cox RE (2009) Does oxytocin influence the early detection of angry and happy faces? Psychoneuroendocrinology 34: 220-225. [Crossref]
- Wilson MR, SJ Vine, Wood G (2009) The influence of anxiety on visual attentional control in basketball free throw shooting. J Sport Exerc Psychol 31: 152-168. [Crossref]
- Murray NP, Janelle CM (2003) Anxiety and performance: A visual search examination of the processing efficiency theory. J Sport Exerc Psychol 25: 171-187
- Schlingensiepen KH, Campbell FW, Legge GE, Walker TD (1986) The importance of eye movements in the analysis of simple patterns. Vision Res 26: 1111-1117. [Crossref]
- Uzzaman S, Joordens S (2011) The eyes know what you are thinking: eye movements as an objective measure of mind wandering. Conscious Cogn 20: 1882-1886. [Crossref]
- Lin JJ, Lin SS (2014) Tracking eye movements when solving geometry problems with handwriting devices. J Eye Mov Res 7
- Lin JJH, Lin SS (2014) Cognitive load for configuration comprehension in computer-supported geometry problem solving: An eye movement perspective. IJSME 12: 605-627.
- Heuer H, Hegele M, Sülzenbrück S (2011) Implicit and explicit adjustments to extrinsic visuo-motor transformations and their age-related changes. Hum Mov Sci 30: 916-930. [Crossref]
- Heuninckx S, Wenderoth N, Swinnen SP (2008) Systems neuroplasticity in the aging brain: recruiting additional neural resources for successful motor performance in elderly persons. J Neurosci 28: 91-99.
- Beurskens R, Bock O (2012) Age-related decline of peripheral visual processing: the role of eye movements. Experimental brain research 217: 117-124.
- Heuer H, Hegele M (2014) Age-related variations of visuo-motor adaptation beyond explicit knowledge. Front Aging Neurosci 6: 152. [Crossref]
- Buch ER, Young S, Contreras-Vidal JL (2003) Visuomotor adaptation in normal aging. Learn Mem 10: 55-63. [Crossref]
- Orrell AJ, Eves FF, Masters RS (2006) Motor learning of a dynamic balancing task after stroke: implicit implications for stroke rehabilitation. Phys Ther 86: 369-380. [Crossref]



