Abstract
Objective: To identify pain prevalence, severity, frequency, duration, quality, location, distribution, type, and treatment in a large, well-designed sample of community dwelling individuals with multiple sclerosis (MS).
Methods: This was a cross-sectional study. A centre-stratified random sample including 188 persons with MS were recruited from three major MS clinics in the Greater Montreal region, Canada. Main outcomes included pain prevalence, severity, frequency, duration, quality, location, distribution, type, and treatment.
Results: 42% identified pain as a symptom, and among those, 60% reported severe pain. Pain differed among participants in severity, type, location, duration, frequency, and quality. Additionally, the average of total percent of body surface that participants had shaded as painful was 20%. Leg pain was the most common anatomical site of pain followed by arm pain and back pain. Neuropathic pain was the most commonly reported type of pain. The majority of participants used pharmacological techniques for pain relief. The pain management techniques were used mainly by women, participants with more disability, severe pain, younger participants, and also those who were employed.
Conclusion: Pain is a common symptom in MS. The considerable severity and distribution of pain on individuals with MS confirm the importance of accurate assessment and adequate intervention approach for pain treatment in people with MS.
Key words:
multiple sclerosis, pain severity, neuropathic pain, pain distribution, pain treatment
Multiple sclerosis (MS) is one of the most disabling chronic demyelinating disease of the central nervous system (CNS) [1]. The exact cause of MS is not known [1-3]. In most people, MS begins between the ages of 20 and 40 and the condition is seen more frequently in women than in men [4,5]. Canada has one of the highest prevalence rates of MS in the world, affecting as manyas 240 people per 100,000. The annual cost of MS has been estimated at $502.3 million in Canada.
Pain is a frequent complaint among individuals with MS [6]. The reported prevalence of pain in MS differs in the literature, ranging from 11% to 90% [6-16]. In Canada, pain has been reported in 41% to 71% of persons with MS [7,16-18]. This variation is due to methodological differences across studies in regards to the patient source, method of sampling, research design, heterogeneity and complexity of the disease itself, and pain measurement.
Pain impacts on several aspects of individuals’ life. In comparison to MS people without pain and the general population, persons with MS pain report poorer health-related quality of life (HRQL), poorer overall mental and general health, more social role limitation [7, 19-22], and more depressive symptoms [23]. Moreover, nearly half of persons with MS and pain report that pain interferes with their daily activities, household work, sleep, and enjoyment of life [8,17,24].
There are substantial gaps in the literature on pain in MS. Nevertheless, pain is very disabling in MS population and is considered to be a major contributor to activity limitations and restrictions in societal and family roles. Available information of MS related pain often is limited by value because of methodological and analytical problems. For the most part, previous studies have looked at pain as a uni-dimensional health outcome or have focused on only few dimensions of pain (mostly intensity and duration) in their analyses. A comprehensive and detailed assessment of pain would help in better understanding of MS pain and result in more targeted treatment approaches for people with MS.
The main objective of the current study, therefore, was to identify pain prevalence, severity, frequency, duration, quality, location, distribution, treatment, and type in a large, well-designed sample of community dwelling individuals with MS.
Materials and methods
Target population was all people with MS, diagnosed since 1995. Available population was all men and women registered at the three major MS clinics in greater Montreal including, Montreal Neurological Hospital (MNH), Centre Hospitalier de l’Université de Montréal (CHUM), and Clinique Neuro Rive-Sud (CNRS). A centre-stratified random sample of 188 (139 women and 49 men) shaped the study sample population.
Eligibility was based on diagnosis of MS or Clinically Isolated Syndrome (CIS). In addition, participants who had a relapse in the preceding month, participants younger than 18 years old, people with severe cognitive impairments, and those with pre-existing health conditions affecting functioning were excluded from participating in the study.
Measures
Socio- demographic factors of gender, age and education level were recorded on the day of testing. In addition, the clinical records and medical charts of each participant were consulted to obtain data on MS type, years since MS diagnosis and symptoms onset, and use of disease modifying therapies (DMT). The severity of neurological impairment was assessed by independent neurologist using the Expanded Disability Status Scale (EDSS) [25].
Pain prevalence
Pain prevalence in persons with MS was determined by calculating the proportion of participants who answered ‘yes’ to this question: “Are you currently experiencing any pain regardless of intensity and localization?” Additional pain questionnaires were only administered to persons who reported pain.
Bodily pain intensity
The two-item bodily pain subscale (BPS) from RAND-36 was used as a measure of bodily pain intensity during the past 4 weeks. The first item of BPS asks about pain intensity, and the second item grades the impact of pain on work. These two items are combined into a single composite score and transformed to a 0-100 scale, with higher scores indicating lower pain severity [26]. Internal consistency and content, criterion and construct validity of RAND-36 have been reported [27-31].
Pain severity
To measure average, lowest and worst pain severity over the previous week as well as pain at the time of evaluation we used 0–10 Numeric Rating Scales (NRS), with 0 indicating ‘No pain’ and 10 indicating ‘the most painful sensation imaginable’. Reliability and validity of NRS have been documented [32]. NRS is also strongly associated with other measures of pain intensity [33-35] and is responsive to changes in pain treatments.
Pain location
To measure pain location, participants were instructed to shade areas that were painful at the time of the evaluation on a pain diagram showing the front and back of the whole body consisting of 45 anatomical areas (Figure 1) [36].
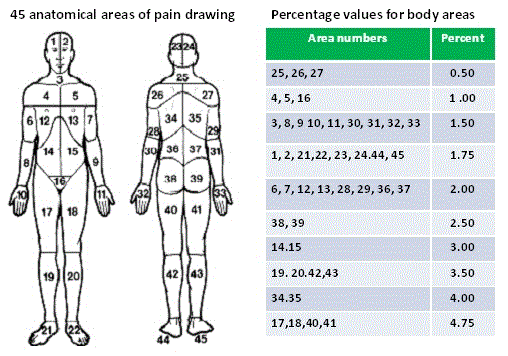
Figure 1. Margolis drawing rating system (Margolis 1986)
Pain distribution
Pain distribution was measured using the Margolis drawing rating system which has 45 anatomical areas each with a corresponding percentage value of body surface in order to compute a total weighted score, indicating body pain distribution (Figure 1) [36]. The test-retest and inter-rater reliability of scale has been established [12].
Pain quality and type
To assess pain quality and type, participants were asked to choose as many as of the words from a list containing 29 adjectives of pain sensation descriptors taken from the McGill Pain Questionnaire (MPQ) [37,38]. Sensations of shooting, stabbing, electric shock-like, nagging, numbness, tingling, and burning were considered as neuropathic pain descriptors, whereas non- neuropathic pain was described as a sharp, aching or throbbing sensation. Superficial pain descriptors included numbness, tingling, burning, shooting, sharp, pressure, piercing, stinging, hot, smarting, radiating, cutting, while deep pain descriptors included cramping, tenderness, aching, pulling, pounding, gnawing, soreness, boring, stabbing, troublesome, annoying, dull, nagging and throbbing.
Pain duration and frequency
Participants were asked to report their pain duration and if their pain experience was constant or not. They were also asked to rate how frequently they experienced pain.
Pain management techniques
Participants were asked to indicate their pain management techniques, either pharmacological or non-pharmacological, during the previous month of the study and to report if these techniques helped relieve their pain. They were also asked to determine which specific medications they took for their pain reduction.
Descriptive statistics (e.g., mean, standard deviations, and frequency) were used to describe the sample and summarize data. The potential for selection bias, differences between responders and non- responders on targeted variables (e.g., socio-demographic and clinical characteristics of persons), and comparison between persons with and without pain was tested using Chi square test for categorical variables, t-test for continuous variables with homogenous variances, and U Mann-Whitney test for continuous variables with non-homogenous variances. Person and spearman correlation coefficient were used to determine the association between study variables. Individuals with missing information from the questionnaire were excluded from the specific analysis. Statistical significance was considered for p-values less than 0.05 Statistical analyses were performed using the Statistical Analysis Systems (SAS) Version 9.2.
Results
Response rate was 52%, and no significant difference was found between responders (n=188) and non-responders (n = 176) on age, sex, MS related disability, date of diagnosis, and duration of symptoms.
Socio- demographic and clinical characteristics of the sample
Socio- demographic and clinical characteristics of the sample are presented in Table 1. The ratio of women to men participants in our study was 3: 1, indicating that women in comparison to men are at higher risk (almost three times) of getting MS. Most participants were receiving DMT at the time of the study. COPAXONE (24%) followed by REBIF (22%) and AVONEX (14%) were the most common types of DMT used by participants.
Pain characteristics of the sample are presented in Table 2. Of the 188 persons, 42% identified pain as a symptom, and among those, 42% reported to have clinically significant pain (severity ≥4) at the time of evaluation. Duration of pain varied. Pain could last from minutes to hours to days. The mean values of bodily pain measured by RAND-36 was 67 ± 27 for the whole sample which is lower than age expected norms of Canadian general population of 76 [39].
The mean value for rating of current pain at the time of evaluation was 3.3 ± 2.3; mean of lowest pain severity was 2.2 ± 2; worst pain severity was 6.8 ± 2; and pain average was 5.0 ± 2. The NRSs later were used to classify the participants as having no pain (score 0), mild pain (scores 1–4), moderate pain (scores 5- 6) and severe pain (scores 7–10) [40,41]. Distribution of the severity of pain is presented in Figure 2. All metrics of patients’ pain ratings were correlated including the calculated average of lowest and worst (Figure 3). In addition, they all correlated similarly with an external pain rating scale (BPS of RAND-36). Interestingly, it was indicated that of all ratings, the patients' ratings of worst pain was the most closely associated with the rating of average pain (r = 0.8).
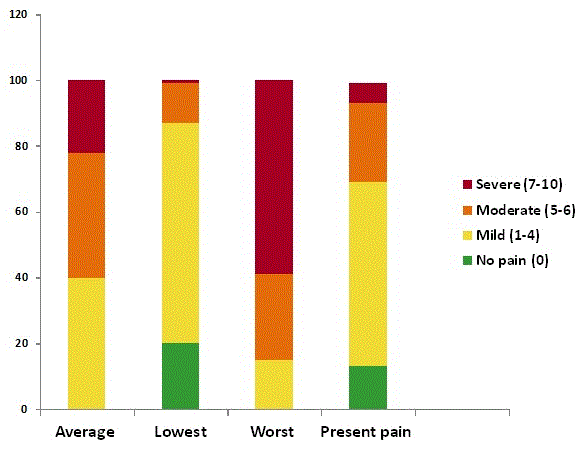
Figure 2. Distribution of pain severity scores within past week (0-10 NRS)
The frequency of pain sites are shown in Table 3. Participants shaded an average of 8 out of 45 parts of body as painful. Leg pain was the most common anatomical site of pain followed by arm pain and back pain. In addition, prevalence of pain was more on the left side than on the right side and in anterior parts rather than posterior parts of the body. Additionally, the average of total percent of body surface that participants had shaded as painful was 20% (range: 2 - 48).
The frequency of the pain descriptors are detailed in Figure 4. The average number of words chosen by participants was 5, and 36% of participants used more than 5 words to describe their pain. Neuropathic pain was the most commonly reported type of pain. There was also a significant association between severity of pain and type of pain, suggesting that neuropathic pain is more severe than non-neuropathic (Fisher exact test, p= 0.03). In addition, we found no statistically significant differences in age and gender between participants with neuropathic and non-neuropathic pain.
Pain management techniques that have been used by participants are presented in Table 4. Overall, 95% of participants reported that the methods they used for pain management, helped with their pain reduction. In addition, there were correlations between gender, age, MS related disability, and employment status with the frequency of using pain management techniques (p <0.05).
As presented in Table 2 there was no difference between 2 groups on age, education, and smoking status, DMT, and duration of symptoms onset and disease diagnosis (p >0.05). However, the pain group showed a higher women-to-men sex ratio (4:1 vs. 2:1 in pain group), higher EDSS scores, and less number of participants with relapsing- remitting MS.
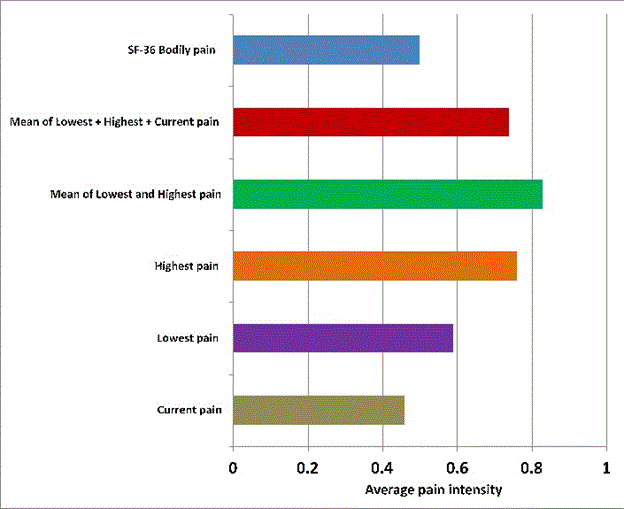
Figure 3. Correlation between average pain severity and pain variables
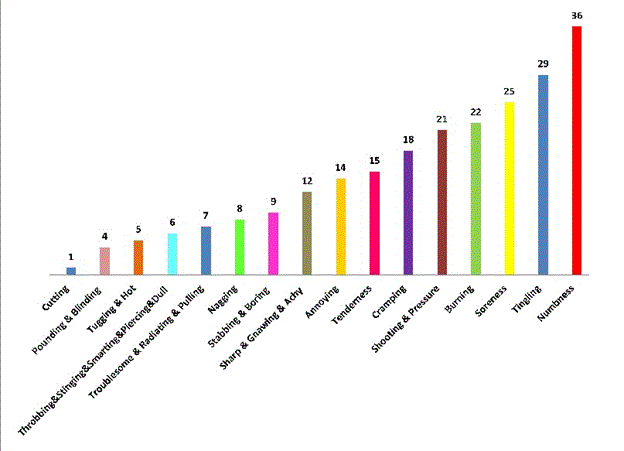
Figure 4. The participants’ descriptions of their abnormal sensation using the words from the McGill Pain Questionnaire
The purpose of the present study was to characterize pain in MS. The prevalence of pain at the time of evaluation was 42% which is located in the range of most reporting rates of 40% to 80% in MS population [7,8,42,43], overlap with 42% in a study conducted by Goodin [42], 43% estimated by Solaro [44], and 41% and % 44 reported by Warnell [16] and Archibald [7] in Canada. However, it is difficult to compare studies because of inconsistencies in measurement, definition of pain, time frame, and variety of patients’ clinical and personal characteristics. One reason for low prevalence of pain in the present study can be related to the fact that our sample had mild disability levels (EDSS< 3) showing the less severely impaired individuals and so less prevalence of symptoms such as pain. However, this emphasizes the need for more attention to pain in MS as it shows that participants, even with low level of disability, had pain. Another reason can be explained by the fact that participants were diagnosed with MS since 1995 while the advances in MS accurate diagnosis e.g. magnetic resonance imaging (MRI) and treatment (e.g. DMT) has changed the clinical course of MS [45,46]. Thus, the MS people diagnosed since 1995 will probably not follow the same symptom patterns and disease course as patients diagnosed before 1995 [47]. The fact that 85% of our participants were using DMT to manage their MS progression and to control their symptoms along with a probable earlier diagnosis of disease, confirm the lower prevalence of pain in our sample.
Mean of average pain severity of our sample was 5 out of 10 which was within the range of 4.6 to 5.8 reported by Douglas [48], Archibald [7], Beiske [49], Heckman- Stone [50], Warnell [16], Rae Grant [43], and Ehde [8]. Participants’ ratings of their worst pain intensity showed that 60% of sample reported severe pain (7-10 out of 10), which is greater than 49% reported in another study [24]. These findings taken together show that despite low prevalence of pain, pain severity was high in our sample, therefore reinforcing the need to identify pain reasons and look for an effective approach to treat it adequately.
Typically in research, pain severity is queried on a 0 to 10 NRS. Research indicates that a single rating of pain severity may not adequately represent the construct of pain [51]. Frequently, multiple pain values are obtained: current, lowest, worst, and average. All of these values are relevant both for patient management and research; but for research, having four values poses logistical and statistical difficulties as several ratings would need either multiple analyses or a different statistical method. Results of this study showed that participants’ estimates of average pain were highly correlated to the calculated average of lowest and worst. Thus, we recommend not asking participants “average” their pain and for research purposes calculate the average of lowest and worst.
Consistent with previous studies, the majority of participants (97%) in this study reported pain in more than one site of their body [6,7,16,17, 22,43,44, 49,48]. Further agreement with other studies was related to the most common site of pain as leg pain had the highest frequency among body segments [7,49,43]. Additionally, we found no relationship among number of pain sites with pain severity and MS disability. These findings confirm the results presented by Archibald [7] and Piwko [18] in Canada.
The average of total percent of body su rface that the participants had shaded as painful was 20% for this sample that was lower than 26.5% reported by Douglas [48]. Results also showed that pain extent was significantly correlated to pain severity, but in contrast with Douglas [48], it was not related to gender. In addition, 55% of participants in our sample reported their pain as intermittent, which is very close to 57% reported by Ehde [17].
With respect to type of pain, most studies on pain characteristics in MS have neither investigated the different types of pain in MS, nor differentiated between neuropathic and non-neuropathic pain. Since each type of pain needs its specific treatment approaches according to its underlying mechanism [52], distinguishing whether pain is neuropathic or not has important treatment relevance. Linked with the results of few other studies [13,15,49], we found that the type of pain in our sample was more often neuropathic than non-neuropathic (25% vs. 9%). Similar to the results of Kalia [10] we also found that neuropathic pain is more severe and disabling pain than non-neuropathic pain. In addition, no statistically significant associations were observed in our study between different forms of pain with age and gender.
In accord with many other studies pain management techniques involved a variety of pharmacologic and non-pharmacologic approaches [7,10,18,48,50,53]; however, similar with results of Archibald [7], Khan [53], the majority of participants used mostly medication. In accord with several other studies such as Khan [53], Kalia [10], Heckman-Stone [50] participants reported that their pain subsided significantly following the use of pain management techniques. Common pain medications used by our sample included opioids and antidepressants which were similar with the findings of Pollman [55]. Non pharmacological techniques commonly used in our sample were massage and exercise which was similar with reports of Kalia [10] and Douglas [48]. The pain management techniques were used mainly by women, participants with more disability, severe pain, younger participants, and also those who were employed. These results were similar with Douglas [48], who found that women and participants in paid employment reported more pain management techniques.
Similar to a previous study [7,8,14,17,44], we found that persons with pain were more likely to have greater MS disability than those without pain. In further agreement with Douglas [48] the present study also found that the greater severity of MS positively correlated with the number of pain locations and pain distribution.
The current study has several strong points. It assessed a variety of MS pain constructs using standardized measures which are often not assessed in MS pain literature. Moreover, in order to limit errors due to memory, the assessment of pain focused on pain experienced over different time frames e.g. current pain and the month and week preceding the assessment. Response rate of study was 52%, very close to the 54% reported by Ehde [8], and higher than the 34% reported by Goodin [42]. The mean age at which participants were diagnosed with MS was 43 years, which corresponds with the results reported by the MS Society of Canada [18]. Additionally, the ratio of women to men in our study was 3:1, which corresponds with the sex ratio of the MS population [55]. 78% of respondents had a relapsing- remitting form of MS which is very close to the prevalence of 75% reported by the Canadian MS Society [18] Also, the study sample was randomly selected from 3 different clinics in Montreal and it included the whole range of MS spectrum. Together all these show the high external validity of study confirming this sample could be a good representative of the general MS population diagnosed since 1995 in Canada.
On the other hand, this study had several limitations. First, this was a cross-sectional study where subjects were assessed at one point in time. This issue is particularly important in MS because as disease progresses, variables contributing to pain could be different. The cross-sectional nature of this data also makes it difficult to accurately examine how the impact of pain changes over time. Longitudinal studies are needed in order to see how the course and severity of MS pain change over time. Additionally, people with the higher pain values might have been reluctant to participate in this study, thus overestimate the results of this study.
In conclusion, results of the current study indicate that pain is a common symptom among people with MS. These findings help us to better understand and characterize the experience of pain among people with MS. Comprehensive assessment of pain in MS would be essential to improve pain treatment.
Acknowledgments
Authors acknowledge the contribution of Élaine Roger and Shang Yuan Teng, who recruited patients from CHUM and MNH, respectively.
Funding
The Canadian Institutes of Health Research
Conflict of interest statement
The Authors declare that there is no conflict of interest.
Ethical considerations
Study protocol and procedures were approved by the ethics committee of each participating hospital, informed consent was obtained and signed by all subjects on the day of testing.
References
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG (2000) Multiple sclerosis. N Engl J Med 343: 938-952. [Crossref]
- Compston A, Confavreux C, Lassmann H (2005) McAlpine's Multiple Sclerosis. Philadelphia: Elsevier.
- Ramagopalan S (2010) Genes and Environmental Factors in Multiple Sclerosis 16: 259-259.
- Orton SM, Herrera BM, Yee IM, Valdar W, Ramagopalan SV, et al. (2006) Sex ratio of multiple sclerosis in Canada: a longitudinal study. Lancet Neurol 5: 932-936. [Crossref]
- Ramagopalan SV, Byrnes JK, Orton SM (2010) Sex ratio of multiple sclerosis and clinical phenotype. European journal of neurology.
- Svendsen KB, Jensen TS, Overvad K, Hansen HJ, Koch-Henriksen N, et al. (2003) Pain in patients with multiple sclerosis: a population-based study. Arch Neurol 60: 1089-1094. [Crossref]
- Archibald CJ, McGrath PJ, Ritvo PG, Fisk JD, Bhan V, et al. (1994) Pain prevalence, severity and impact in a clinic sample of multiple sclerosis patients. Pain 58: 89-93. [Crossref]
- Ehde DM, Gibbons LE, Chwastiak L, Bombardier CH, Sullivan MD, et al. (2003) Chronic pain in a large community sample of persons with multiple sclerosis. Mult Scler 9: 605-611. [Crossref]
- Indaco A, Iachetta C, Nappi C, Socci L, Carrieri PB (1994) Chronic and acute pain syndromes in patients with multiple sclerosis. Acta Neurol (Napoli) 16: 97-102. [Crossref]
- Kalia LV, O'Connor PW (2005) Severity of chronic pain and its relationship to quality of life in multiple sclerosis. Mult Scler 11: 322-327. [Crossref]
- Kassirer MR, Osterberg DH (1987) Pain in multiple sclerosis. Am J Nurs 87: 968-969. [Crossref]
- Margolis RB, Chibnall JT, Tait RC (1988) Test-retest reliability of the pain drawing instrument. Pain 33: 49-51. [Crossref]
- Moulin DE (1998) Pain in central and peripheral demyelinating disorders. Neurol Clin 16: 889-898. [Crossref]
- Stenager E, Knudsen L, Jensen K (1991) Acute and chronic pain syndromes in multiple sclerosis. Acta Neurol Scand 84: 197-200. [Crossref]
- Vermote R, Ketelaer P, Carton H (1986) Pain in multiple sclerosis patients: a prospective study using the McGill pain questionnaire. Clin Neurol Neurosurg 88: 87-93.
- Warnell P (1991) The pain experience of a multiple sclerosis population: a descriptive study. Axone 13: 26-28. [Crossref]
- Ehde DM, Osborne TL, Hanley MA, Jensen MP, Kraft GH (2006) The scope and nature of pain in persons with multiple sclerosis. Mult Scler 12: 629-638. [Crossref]
- Piwko C, Desjardins OB, Bereza BG, Machado M, Jaszewski B, et al. (2007) Pain due to multiple sclerosis: analysis of the prevalence and economic burden in Canada. Pain Res Manag 12: 259-265. [Crossref]
- Murphy DF, McDonald A, Power C, Unwin A, MacSullivan R (1988) Measurement of pain: a comparison of the Visual Analogue Scale with a Nonvisual Analogue Scale. Clin J Pain 3: 197-199.
- Nortvedt MW, Riise T, Myhr KM, Nyland HI (1999) Quality of life in multiple sclerosis: measuring the disease effects more broadly. Neurology 53: 1098-1103. [Crossref]
- Shahrbanian Sh, Auais M, Duquette P, Anderson K, Mayo NE (2013) Does Pain in Individuals with Multiple Sclerosis Affect Employment? A Systematic Review and Meta-analysis. Pain Res Manag 18: e94-e100.
- Svendsen KB, Jensen TS, Hansen HJ, Bach FW (2005) Sensory function and quality of life in patients with multiple sclerosis and pain. Pain 114: 473-481. [Crossref]
- Ehde DM, Osborne TL, Jensen MP (2005) Chronic Pain in Persons with Multiple Sclerosis. Physical Medicine and Rehabilitation Clinics of North America 16: 503-512.
- Hadjimichael O, Kerns RD, Rizzo MA, Cutter G, Vollmer T (2007) Persistent pain and uncomfortable sensations in persons with multiple sclerosis. Pain 127: 35-41. [Crossref]
- Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33: 1444-1452. [Crossref]
- Hays RD, Morales LS (2001) The RAND-36 measure of health-related quality of life. Ann Med 33: 350-357. [Crossref]
- Brunet DG, Hopman WM, Singer MA, Edgar CM, MacKenzie TA (1996) Measurement of health-related quality of life in multiple sclerosis patients. Can J Neurol Sci 23: 99-103. [Crossref]
- Brazier JE, Harper R, Jones NM, O'Cathain A, Thomas KJ, et al. (1992) Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ 305: 160-164. [Crossref]
- Freeman JA, Hobart JC, Langdon DW, Thompson AJ (2000) Clinical appropriateness: A key factor in outcome measure selection: the 36 item short form health survey in multiple sclerosis. Journal of Neurology and Neurosurgery 68: 150-156.
- Katz JN, Larson MG, Phillips CB, Fossel AH, Liang MH (1992) Comparative measurement sensitivity of short and longer health status instruments. Medical Care 30: 917-925.
- McHorney CA, Ware JE, Jr. Raczek AE (1993) The MOS 36-Item Short-Form Health Survey (SF-36): II. A psychometric and clinical test of validity in measuring physical and mental health constructs. Medical Care 31: 247-263.
- Sharrack B, Hughes RA, Soudain S, Dunn G (1999) The psychometric properties of clinical rating scales used in multiple sclerosis. Brain 122: 141-159. [Crossref]
- Jensen MP, Karoly P, Braver S (1986) The measurement of clinical pain intensity: a comparison of six methods. Pain 27: 117-126. [Crossref]
- Jensen MP, Karoly P, Harris P (1991) Assessing the affective component of chronic pain: development of the Pain Discomfort Scale. J Psychosom Res 35: 149-154. [Crossref]
- Jensen MP, Turner JA, Romano JM, Fisher LD (1999) Comparative reliability and validity of chronic pain intensity measures. Pain 83: 157-162. [Crossref]
- Margolis RB, Tait RC, Krause SJ (1986) A rating system for use with patient pain drawings. Pain 24: 57-65. [Crossref]
- Melzack R (1983) The McGill Pain Questionnaire. In: Melzack R, (Ed.,s) Pain measurement and assessment. New York: Raven pp :41-47.
- Melzack R (1987) The short-form McGill Pain Questionnaire. Pain 30: 191-197. [Crossref]
- Hopman WM, Towheed T, Anastassiades T, Tenenhouse A, Poliquin S (2000) Canadian normative data for the SF-36 health survey. Canadian Medicine Association Journal 163: 265-271.
- Grasso MG, Clemenzi A, Tonini A, Pace L, Casillo P, et al. (2008) Pain in multiple sclerosis: a clinical and instrumental approach. Mult Scler 14: 506-513. [Crossref]
- Serlin RC, Mendoza TR, Nakamura Y, Eduards KR (1995) When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain 61: 277-284.
- Goodin DS (1999) Survey of multiple sclerosis in northern California. Northern California MS Study Group. Mult Scler 5: 78-88. [Crossref]
- Rae-Grant AD, Eckert NJ, Bartz S, Reed JF (1999) Sensory symptoms of multiple sclerosis: a hidden reservoir of morbidity. Mult Scler 5: 179-183. [Crossref]
- Solaro C, Brichetto G, Amato MP, Cocco E, Colombo B, et al. (2004) The prevalence of pain in multiple sclerosis: a multicenter cross-sectional study. Neurology 63: 919-921. [Crossref]
- Kieseier BC, Hartung HP (2003) Current disease-modifying therapies in multiple sclerosis. Semin Neurol 23: 133-146. [Crossref]
- Massimo F, Rohit B, Marco R, Giancarlo C (2007) MRI and Multiple Sclerosis: What Happened in the Last 10 Years. Journal of Neuroimagaing 17: 1S-2S.
- Mayo N (2008) Setting the agenda for multiple sclerosis rehabilitation research. Mult Scler 14: 1154-1156. [Crossref]
- Douglas C, Wollin JA, Windsor C (2008) Illness and demographic correlates of chronic pain among a community-based sample of people with multiple sclerosis. Arch Phys Med Rehabil 89: 1923-1932.
- Beiske AG, Pedersen ED, Czujko B, Myhr KM (2004) Pain and sensory complaints in multiple sclerosis. Eur J Neurol 11: 479-482. [Crossref]
- Heckman-Stone C, Stone C (2001) Pain management techniques used by patients with multiple sclerosis. J Pain 2: 205-208. [Crossref]
- Spadoni GF, Stratford PW, Solomon PE, Wishart LR (2004) The evaluation of change in pain intensity: a comparison of the P4 and single-item numeric pain rating scales. J Orthop Sports Phys Ther 34: 187-193. [Crossref]
- Moulin DE, Foley KM, Ebers GC (1988) Pain syndromes in multiple sclerosis. Neurology 38: 1830-1834. [Crossref]
- Khan F, Pallant J (2007) Chronic Pain in Multiple Sclerosis: Prevalence, Characteristics, and Impact on Quality of Life in an Australian Community Cohort. The Journal of Pain 8: 614-623.
- Pollman W, Feneberg W, Steinbrecher A, Haupts M, Henze T (2005) Therapy of pain syndromes in multiple sclerosis: An overview with evidence-based recommendations. Fortschr Neurol Psychiatr 73: 268-85.
- O'Connor P (2002) Canadian Multiple Sclerosis Working Group -Key issues in the diagnosis and treatment of multiple sclerosis. An overview. Neurology 59: S1-33. [Crossref]




