Abstract
Cerebral Palsy (CP) is one of the most2021 Copyright OAT. All rights reservd it demands important costs in health, education, and social services. CP is caused by damage to or abnormalities inside the developing brain that disrupt the brain's ability to control movement and maintain posture. Furthermore, CP is often associated with sensory deficits, cognition impairments, communication and motor disabilities, behavior issues, seizure disorder, pain, and secondary musculoskeletal problems. According to the literature, motor modules are peripheral measurements related to automatic motor control. There is a lack of evidence of change in motor modules in children with CP when different treatment approaches have been evaluated. Thus, new strategies are needed to improve motor control in this population.
Robotic-based therapies are emerging as an effective intervention for gait rehabilitation in motor disorders such as stroke, spinal cord injury, and CP. There is vast clinical evidence that neural plasticity is the central core of motor recovery and development, and on-going studies suggest that robot-mediated intensive therapy could be beneficial for improved functional recovery. However, current robotic strategies are focused on the peripheral neural system (PNS) facilitating the performance of repetitive movements (a bottom-up approach). Since CP affects primarily brain structures, both the PNS and the central nervous system (CNS) should to be integrated in a physical and cognitive rehabilitation therapy (a top-down approach).
This paper discusses perspectives of the top-down approach based on a novel robot-assisted rehabilitative system. Accordingly, the CPWalker robotic platform was developed to support novel therapies for CP rehabilitation. This robotic platform (Smart Walker + exoskeleton) is controlled by a multimodal interface enabling the interaction of CP infants with robot-based therapies. The aim of these therapies is to improve the physical skills of infants with CP using a top-down approach, in which motor related brain activity is used to drive robotic physical rehabilitation therapies. Our hypothesis is that the CPWalker concept will promote motor learning and this improvement will lead to significant improvements in automatic motor control.
Key words
cerebral Palsy, rehabilitation robotics, top-down approach, motor control, gait
Introduction
Cerebral Palsy (CP) is a term that could be defined as ‘a persisting but not unchanging disorder of movement and posture, appearing in the early years of life and due to a non-progressive disorder of the brain, the result of interference during its development’ [1,2]. CP is often associated to sensory deficits, cognition impairments, communication and motor disabilities, behavior issues, seizure disorder, pain, and secondary musculoskeletal problems. Furthermore, CP is an important cause of permanent serious physical disability in childhood, and in recent years, the survival rates of children with severe level of disability has increased [3]. Consequently, more and more children with CP learn to walk with abnormal patterns, and this inefficient gait is accentuated as the child grows and increases weight.
In some cases, the development of secondary musculoskeletal pathology contributes to loss of function, gait impairments, fatigue, activity limitations, and participation restriction. One of the best treatments used in musculoskeletal impairment in CP isorthopedic surgery with its main technique being single-event multilevel orthopedic surgery (SEMLS) [4]. This surgical procedure consists of two or more soft-tissue or bony surgical techniques at two or more anatomical levels during one sole operative procedure [5]. After this procedure, a period of 2 years is often required in order to obtain an appropriate functional level. Consequently, new strategies are needed to help to promote, maintain, and rehabilitate the functional capacity, and thereby diminish the dedication and assistance required, and the economical demands that this condition represents for the patient, the caregivers, and society at large [6].
Experimental evidence suggests that diverse human motor behaviors are constricted by a combination of rudimentary motor modules, which probably reside at the spinal cord or mesencephalic centers. Each motor module involves a basic activation pattern with variable weights of distribution to different muscles [7,8]. In this sense, the concept of muscle synergy has emerged in neuroscience and has been proposed as a mechanism for neural control of normal movement [9]. Muscle synergies in healthy subjects represent functional muscle coordination patterns used to reliably produce motor functions during natural motor behaviors (4-5 synergies for gait) [7,9,10]. Interestingly, a reduced number of synergies during gait have been reported for children with CP (2-3 synergies). Moreover, no changes have been observed that derive from therapeutic approaches introduced for management of gait disorders [12,13].
The integration of intense, task-related exercise strategies, and the comprehensive combination of non-invasive treatment, surgical interventions, and new technologies (e.g. robotic platforms) has been proposed to improve current rehabilitation strategies [14]. According to a bi-center survey [15], no severe side effects have emerged from these strategies and the vast majority of children were highly motivated to participate with motivation being sustained throughout the training period. Moreover, clinical experience suggests that gait training in children with considerable cognitive deficits could be conducted even more effectively using robotic-assisted therapy rather than conventional over-ground training [16].
Traditional robotic platforms, such as the Lokomat (Hocoma AG, Switzerland), use strategies which are based on motor control reorganization triggered by peripheral physical therapy (a bottom-up approach). However, CP affects primarily brain structures, suggesting that both the peripheral nervous system (PNS) and the central nervous system (CNS) should be integrated into physical and cognitive rehabilitation therapy [17]. Current studies suggest that such integration of the CNS into the human-robot loop maximizes the therapeutic effects, especially in infants, as it enhances therapy adherence. This approach is known as a top-down approach [17]: motor patterns of the limbs are represented in the cortex, transmitted to the limbs, and fed back to the cortex. Although this approach has been previously studied in other populations (e.g. stroke [18]), there is a lack of studies in CP [19]. It is important to highlight that young children, target patients of the present proposal, present increased brain plasticity compared to adult patients, and that they are more likely to change motor patterns following an intervention.
In support of the use of the CPWalker as a novel approach for CP rehabilitation, a validation study is further proposed. CPWalker offers the use of a robotic platform controlled by the user (top-down approach) through which the infant can start experiencing autonomous locomotion in a rehabilitation environment. The interaction between the infant and the robotic platform takes place through a multimodal human-robot interface (MHRI). This MHRI consists of an electroencephalographic (EEG) acquisition unit and an electromyography (EMG) system for measuring residual movement and activation strategies. The rationale of this multimodal interface is to allow integrated PNS and CNS physical and cognitive interventions. MHRI interaction with therapeutically selected tasks will be based on volitional motor planning, the aim being promoting re-organization of motor planning brain structures and thus integrating CNS in the therapy.
This highly cross-disciplinary research represents a unique framework that will allow us to collect never before captured behavioral and neurophysiological data from children with CP, which will offer a deeper theoretical insight on the effects that robotic-assisted therapies have on pathological pediatric gait at an individual level.
Finally, active participation during rehabilitation sessions is considered a key-factor in order to achieve a good motor outcome. This is supported by the fact that motivation scores of children with CP have been reported to be much higher on dimensions of mastery pleasure and social persistence than on dimensions of persistence with cognitive or motor tasks for which motivation scores were lowest, indicating difficulty to remain motivated during prolonged duration of motor rehabilitation programs [20]. The top-down approach proposed in our study intends to address this issue by promoting the active participation of the patient during the therapy [18].
Methods
Provisional experimental setup
The experimental setup is based on the robotic platform CPWalker, which is composed by the following subsystems: 1) a smart walker with body weight support, 2) a wearable exoskeleton robot (ER), 3) a MHRI and 4) a software analysis tool (Figures 1and 2). Based on this approach, CPWalker will provide a new concept in physical and cognitive therapies for CP infants, which will allow integrated PNS and CNS physical and cognitive interventions. This will be achieved in many ways:
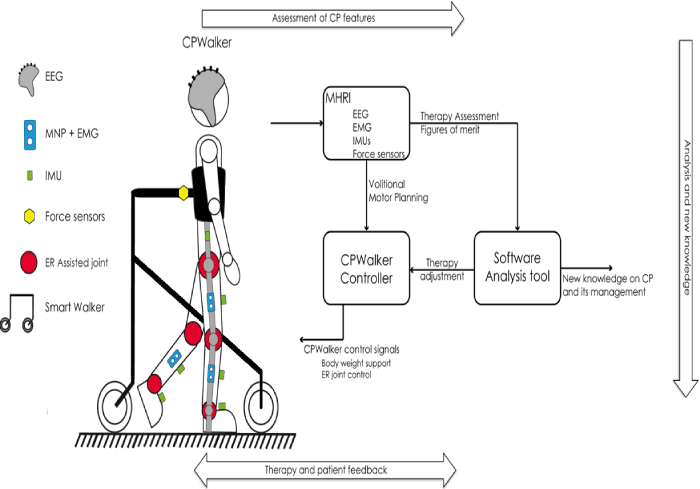
Figure 1. Diagram of the CPWalker robotic platform

Figure2. Prototype of the CPWalker robotic platform and the exoskeleton designed for the platform
- Specific therapies will be developed in which motor planning and brain reorganization is promoted both by PNS physical therapies in combination with MHRI data related to execution tasks. These therapies will be implemented through the robotic platform developed. For these therapies, the MHRI system will be used instead to derive information on motor planning and motor imagery. This information will in turn be used to drive PNS physical therapies, e.g. repetitive assisted active joint movements, so that a link between central and peripheral motor mechanisms is initiated and promoted. This will improve current therapies for CP rehabilitation.
- Both the body position and the movements carried out by the child will be analyzed by a multiple approach based on the use of EMG, inertial movement units (IMU), and force sensors integrated into the robotic platform. This will provide both kinematic and kinetic information of the executed tasks. While the IMU sensors will be related to the analysis of the residual movements and posture, the EMG system will be set up in order to detect muscular activation strategies, and to analyze how they are modified during the rehabilitation treatment. The force sensors will measure the interaction forces between the user and the robotic plattform. Since the acquisition of motor skills is connected to the appearance of the so called muscular synergies, this information will grant an accurate estimation of the muscular strategies actuated by the child. It will be possible to have a quantitative evaluation of the rehabilitation progress, measuring e.g. the timing and amplitude of co-contraction for the muscles involved in the movements executed by the child as well as the development of new coordination patterns for locomotion.
- The system will be also used to provide both a simplified real time biofeedback to the infants, and an off-line report to therapists and care-givers on therapy progress and motor evolution of CP infants. Information for feedback will be derived from the MHRI system, e.g. trends in involuntary movements like spasms and spasticity as well as time and effort during motor planning; and from robot information, e.g. trajectories and driving time.
The clinical trial will be designed to test the superiority of the combination of robotic-assisted therapy with MHRI interface, as compared with the same therapy driven peripherally in the recovery process of gait in children with CP. Subjects will be randomized according to a block randomization to receive robotic training with MHRI or robotic training without MHRI. Each subject will receive daily CPWalker training sessions over three weeks, five sessions per week (workdays), 45 to 60 minutes per session. A qualified member of the study staff will always be present through the entire session. The proposed clinical trials will be based on CPWalker robotic platform and on standard scales of common use in clinical practice and clinical rehabilitation settings, therefore the protocols during experimentation will be close to clinical setting replication.
Hypothesis
The concept proposed in this study aims to reduce the rehabilitation period by the use of an intensive rehabilitation program based on robotics and a top-down approach, which will improve motor control of children with CP. The hypothesis presented in this paper is based on the increased brain plasticity and the crucial role of motor learning trained with the CPWalker robotic rehabilitative platform. This hypothesis relies on the following assumptions:
- Robot-assisted gait trainers can deliver an intensive therapy and provide objective measures of the patient's performance. Moreover, performing gait in real ambulatory scenarios can be a more challenging situation in which the movement is developed. There is evidence that promoting the earlier incorporation of CP patients to the rehabilitation therapy, increasing the level of intensity and frequency of the exercises, and enabling the maintenance of therapeutic methods on a daily basis leads to significant improvements in the treatment outcome.
- Early use of assistive technology in children with CP can be of paramount importance. It is during early stages of development that fundamental abilities and skills are developed. It is also at this stage when corporal, spatial, and time structures are integrated in the cognitive development. From the birth of the child, cognitive skills are developed through interrelation and interaction with the environment through autonomous locomotion. These early stages are also characterized by a high degree of flexibility and plasticity both in physiological and psychological structures, rendering a maximum capability of rehabilitation.
- The rehabilitation protocol is an intensive task-oriented program focused on the main gait impairments obtained form 3D gait analysis data and the Gross Motor Function Classification System functional test (GMFCS). Motor imaginery is used as a preparation tool for children and as a biofeedback tool during therapy. The rehablilitation platform will be triggered using EEG information. The EMG, IMU, and robotic joints will give a continuous feedback to the robotic platform and the clinician in order to optimize the gait cycle and adjust speed, body-weigth support, and range of motion of the different joints. This retroalimentation loop is the basis of the neuroprothesis, mainly working as propioceptive input for the platform giving a real time opportunity to change motor planning and improve motor learning. It will serve as the local control for the biomechanics of the exoskeleton and the Smart Walker. Children will receive an auditory feed-back when the therapy goals are attained during the training. This will reinforce the motivation required for promoting neuroplasticity. A parallell gamification will be designed for the control of evolution and children participation in therapy planning.
Discussion and conclusions
This study proposes a new therapeutical approach to accelerate and improve the rehabilitation process in children with CP through the use of a novel ambulatory robotic gait trainer. The CPWalker enables the implementation of strategies for both ambulatory bodyweight-supported training and constraint induced movement therapy, in order to promote changes in the affected brain structures that will trigger normalized gait patterns. The sensor equipped platform will also enable the study of the reorganization of the muscle activation patterns (at the CNS and PNS) as a means to objectively assess the outcome of the therapy, and also help to elucidate the neural mechanisms that mediate such recovery.
This project builds on vast previous clinical evidence that neural plasticity is the central core of motor development, and on studies suggesting that robot-mediated intensive therapy is beneficial for improved functional recovery [18]. These approaches need to be refined and critically analyzed to determine their functional benefit for children with different levels of sensory-motor or cognitive impairment or both.
Current level of evidence regarding the efficacy of new technologies in the rehabilitation process still remains scarce. Novel studies must involve follow-up measurements to determine if gains will have long-term and lasting impact for children with CP. The platform presented and the way we proposed for its use in clinical practice will allow authors to implement such study and precisely evaluate the effects of different control strategies on this population. The assessment will be carried out based on objective measures derived from the information of the MHRI.
A randomized clinical trial must be conducted for the clinical validation of the robotic rehabilitation platform. We propose to compare robotic training with MHRI against robotic training without MHRI[21]. In addition, specific objective and subjective assessment tools must be addressed in order to build a novel rehabilitation strategy [22-25].
Improvements in motor control cannot be reached without the basic pieces of learning. Thus, the CPWalker concept was designed for creating a repetitive task-oriented training, looking for a strong alliance of motivation and repetition as neuroplasticity triggers [21,26,27].
In conclusion, the prototype described has been designed to introduce novel rehabilitation therapies in the treatment of children with CP. The results obtained with the future clinical validation will provide important outcomes to justify the use of top-down approach based robotic therapy.
Acknowledgement
The work presented in this paper has been carried out with the financial support from the Ministerio de Economía y Competitividad, under Contract DPI2012-39133-C03-01, “CPWalker - Robotic Platform for Gait Rehabilitation and Training in patients with Cerebral Palsy”.
References
- Bax M, Goldstein M, Rosenbaum P, Leviton A, Paneth N, et al. (2005) Proposed definition and classification of cerebral palsy, April 2005. Dev Med Child Neurol47:571-576. [Crossref]
- (2002) Prevalence and characteristics of children with cerebral palsy in Europe. Dev Med Child Neurol44: 633-640. [Crossref]
- Winter S, Autry A, Boyle C, Yeargin-Allsopp M (2002) Trends in the prevalence of cerebral palsy in a population-based study. Pediatrics110: 1220-1225. [Crossref]
- Blair E (2010) Epidemiology of the cerebral palsies. OrthopClin North Am41: 441-455. [Crossref]
- McGinley JL, Dobson F, Ganeshalingam R, Shore BJ, Rutz E, et al. (2012) Single-event multilevel surgery for children with cerebral palsy: a systematic review. Dev Med Child Neurol 54: 117-128. [Crossref]
- Thomason P, Baker R, Dodd K, Taylor N, Selber P, et al. (2011) Single-event multilevel surgery in children with spastic diplegia: a pilot randomized controlled trial. J Bone Joint Surg Am93: 451-460. [Crossref]
- Parkes J, Caravale B, Marcelli M, Franco F, Colver A (2011) Parenting stress and children with cerebral palsy: a European cross-sectional survey. Dev Med Child Neurol53: 815-821. [Crossref]
- Dominici N, Ivanenko YP, Cappellini G, d'Avella A, Mondì V, et al. (2011) Locomotor primitives in newborn babies and their development. Science334: 997-999. [Crossref]
- Clark DJ, Ting LH, Zajac FE, Neptune RR, Kautz SA (2010) Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J Neurophysiol103: 844-857. [Crossref]
- Safavynia SA, Torres-Oviedo G, Ting LH (2011) Muscle Synergies: Implications for Clinical Evaluation and Rehabilitation of Movement. Top Spinal Cord InjRehabil17: 16-24. [Crossref]
- Torres-Oviedo G, Ting LH (2010) Subject-specific muscle synergies in human balance control are consistent across different biomechanical contexts. J Neurophysiol103: 3084-3098. [Crossref]
- Steele KM, Rozumalski A, Schwartz MH (2015) Muscle synergies and complexity of neuromuscular control during gait in cerebral palsy. Dev Med Child Neurol57: 1176-1182. [Crossref]
- Loma-OssorioGarcía M, Torricelli D, Moral Saiz B, Parra Mussin EM, Martín Lorenzo T, et al. (2015) Changes in Modular Control of Gait following SEMLS in Children with Cerebral Palsy. Gait Posture42-56.
- Damiano DL, Alter KE, Chambers H (2009) New clinical and research trends in lower extremity management for ambulatory children with cerebral palsy. Phys Med RehabilClin N Am20: 469-491. [Crossref]
- Borggraefe I, Klaiber M, Schuler T, Warken B, Schroeder SA, et al. (2010) Safety of robotic-assisted treadmill therapy in children and adolescents with gait impairment: a bi-centre survey. DevNeurorehabil13: 114-119. [Crossref]
- Fasoli SE, Ladenheim B, Mast J, Krebs HI (2012) New horizons for robot-assisted therapy in pediatrics. Am J Phys Med Rehabil 91: S280-289. [Crossref]
- Belda-Lois JM, Mena-del Horno S, Bermejo-Bosch I, Moreno JC, Pons JL, et al. (2011) Rehabilitation of gait after stroke: a review towards a top-down approach. J NeuroengRehabil8: 66. [Crossref]
- Lo AC, Guarino PD, Richards LG, Haselkorn JK, Wittenberg GF, et al. (2010) Robot-assisted therapy for long-term upper-limb impairment after stroke. N Engl J Med 362: 1772-1783. [Crossref]
- Meyer-Heim A, van Hedel HJ (2013) Robot-assisted and computer-enhanced therapies for children with cerebral palsy: current state and clinical implementation. SeminPediatrNeurol20: 139-145. [Crossref]
- Majnemer A, Shevell M, Law M, Poulin C, Rosenbaum P (2010) Level of motivation in mastering challenging tasks in children with cerebral palsy. Dev Med Child Neurol52: 1120-1126. [Crossref]
- Sukal-Moulton T, Clancy T, Zhang LQ, Gaebler-Spira D (2014) Clinical application of a robotic ankle training program for cerebral palsy compared to the research laboratory application: does it translate to practice? ArchPhys Med Rehabil95: 1433-1440. [Crossref]
- Zwicker JG, Mayson TA (2010) Effectiveness of treadmill training in children with motor impairments: an overview of systematic reviews. PediatrPhysTher22: 361-377. [Crossref]
- Péter O, Fazekas G, Zsiga K, Dénes Z (2011) Robot-mediated upper limb physiotherapy: review and recommendations for future clinical trials. Int J Rehabil Res34: 196-202. [Crossref]
- Sandin KJ (2012) New approaches to neurorehabilitiation: the increasing evidence base. Minn Med 95: 46-48. [Crossref]
- Luft AR (2012) How to gain evidence in neurorehabilitation: a personal view. Biomed Tech (Berl)57: 427-433. [Crossref]
- Pelletier SJ, Cicchetti F (2014) Cellular and molecular mechanisms of action of transcranial direct current stimulation: evidence from in vitro and in vivo models. Int J Neuropsychopharmacol18. [Crossref]
- Inguaggiato E, Sgandurra G, Perazza S, Guzzetta A, Cioni G (2013) Brain reorganization following intervention in children with congenital hemiplegia: a systematic review. Neural Plast2013: 356275. [Crossref]


