Abstract
Background: Providing an adequate caloric intake is essential for improving clinical outcomes, because inadequate nutrition induces several problems. Previous studies have shown that energy expenditure calculated by classical prediction equations, such as the Harris-Benedict equation (HBE), is greater than the measured resting energy expenditure (REE). To compensate for this discrepancy, additional factors, such as a stress factor, are often used to rescale the value. However, the numerical value of the stress factor, particularly in septic patients, is unknown. Indirect calorimetry (IC) enables to the real-time measurement of REE at the bedside. We hypothesized that the stress factor could decrease over the time course of recovery in septic patients. To test this hypothesis, we measured the REE of septic patients hospitalized in our ICU throughout their intubation period.
Methods: This single-center retrospective study was conducted to compare resting REE measured by IC and the estimated energy expenditure in adult septic patients. Basal metabolic rate (BMR) was estimated by the HBE. Chronological changes in the ratio of measured REE to BMR were analyzed to estimate the stress factor.
Results: A total of 47 patients with sepsis were included in this study. We found that REE/BMR in the septic patients was in the range of 1.07 to 1.11. Moreover, the estimated stress factor did not change over time during the intubation period. REE/BMR did not depend on the number of sedatives administered. Both sequential organ failure assessment (SOFA) scores and blood concentrations of C-reactive protein (CRP) decreased over time. Respiratory quotient (RQ) on the last intubation day was greater than that on the first day.
Conclusions: The REE measured by IC in sedated septic patients was approximately 1.1 times greater than BMR. The ratio of measured REE to BMR does not change with resolution of the illness. This result was likely caused by the concomitant increase in energy intake and improvement in patient general condition at this time. These findings may contribute to better nutrition control in ICU-admitted septic patients.
Key words
indirect calorimetry, resting energy expenditure, sepsis, Harris-Benedict equation
Introduction
Providing an adequate caloric intake to hospitalized patients is essential for improving clinical outcomes. Overfeeding, for example, could induce several problems, including hyperglycemia, hypercapnia, azotemia, and immune deficiency [1-3]. Therefore, predicting total energy requirements is important for preventing these complications. To predict energy requirements, numerous mathematical prediction equations, such as Harris-Benedict, Schofield, Ireton-Jones, Penn State, and Swinamer equations, have been developed [4-8]. Although the Harris-Benedict equation (HBE) is the oldest of these equations, published in 1919, due to its simplicity it still plays a major role in nutrition management in clinical settings. The HBE was developed based on data collected from a population of healthy volunteers [4]. To apply the HBE to hospitalized patients, additional factors, such as stress and activity factors, are often incorporated into the equation to account for the elevated energy expenditure due to stress or injury [9,10]. As a stress factor for critically ill patients, for example, a value between 1.2 and 1.6 has been chosen in past studies [11-15]. However, few studies have reported on the number that should be used as the stress factor in septic patients [16,17].
Release of proinflammatory cytokines and stress hormones in septic patients results in several metabolic changes, including an increase in energy requirements. The secreted cytokines and stress hormones catabolize skeletal muscle and body fat, and the resultant catabolites are utilized as endogenous energy substrates. Particularly in the early clinical stage of sepsis, an increase in total energy expenditure (TEE) is expected as a result of increase in endogenous energy supply due to enhanced catabolism [18].
Indirect calorimetry (IC) is currently considered the most accurate method for measuring caloric needs [19,20]. IC measures oxygen consumption and carbon dioxide excretion, which are used to calculate the respiratory quotient (RQ) and resting energy expenditure (REE), using the Weir equation [21]. We hypothesized that the stress factor in septic patients could be calculated by dividing the value of REE by the basal metabolic rate (BMR) that is calculated using prediction equations.
Further, the stress factor that should be adopted for septic patients could change over the course of the patient’s illness, since the total amount of released cytokines and stress hormones depends on the severity of the illness. We hypothesized that the stress factor could become smaller during recovery in septic patients, because REE probably decreases with resolution of the illness. To test this hypothesis, we measured REE in septic patients hospitalized in our ICU and investigated the changes in REE/BMR over the period during which they were intubated.
Methods
Study design
This retrospective observational study was conducted at the intensive care unit (ICU) of Gunma University Hospital. This study was approved by the institutional ethics committee of our facility. Moreover, information was published on the web page of our hospital to inform patients about the study protocol, and give them a chance to refuse inclusion in the study. Adult patients (≥18 years of age) admitted to the ICU with a diagnosis of sepsis between April 2010 and March 2015 and who were mechanically ventilated for over three days were included. All the patients included in the study fulfilled the diagnostic criteria for severe sepsis [22,23]. Mechanically ventilated patients who met one or more of the following criteria were excluded: fraction of inspired oxygen (FiO₂) ≥ 0.6, positive end expiratory pressure (PEEP) >12 cmH2O, respiratory rate >35 breaths/min, and presence of a chest drain with leakage. In addition, patients on hemodialysis or continuous renal replacement therapy were excluded.
Data collection
We measured REE using a mechanical ventilator with an in-built IC (Engström Carestation®, GE Healthcare Japan). This ventilator automatically calculated REE using the Weir equation.
Weir equation [21]
REE (kcal/day) = (3.94 × VO₂ + 1.10 × VCO₂) × 1.44 − (2.17 × UN*)
VO₂: oxygen consumption (mL/min), VCO₂: carbon dioxide production (mL/min), UN: urinary nitrogen excretion (g). * In the Engström Carestation®, the value of UN is fixed at 13 g/day.
RQ (Respiratory quotient) = VCO₂/VO₂, was also assessed.
The ventilator with its data migration system enables continuous monitoring of REE. We selected the data measured at 2 a.m. on the first, second, and last days of the intubation period. The protocol required (1) that patients be inactive and undisturbed for 30 minutes before testing and for the 15-minute duration of data collection, (2) an interval of at least 30 minutes between changes in ventilator settings and measurements, and (3) an interval of at least 4 hours between changes in the feeding method and measurements. When the RQ was less than 0.67 or greater than 1.3, we discarded the values and instead incorporated the data obtained as close to 2 a.m. as possible [24-28]. We used any one or more of the following sedatives, as required: propofol, dexmedetomidine, midazolam, and fentanyl (Supplemental Table S1). Predicted BMR was calculated by either the Harris-Benedict or Schofield equation using actual body weight and height on ICU admission.
Table S1. Demographic data of patients, resting energy expenditure, calculated energy expenditure, APACHE II score, blood concentration of CRP, SOFA score, respiratory quotient, energy intake, and sedative usage in the study subjects.
|
number
|
age
(years)
|
sex
|
disease
|
intubated
days
|
height
(cm)
|
weight
(kg)
|
BMI
(kg/m²)
|
HBE
(kcal)
|
Schofield
(kcal)
|
REE
1st intubated day (kcal)
|
REE
2nd intubated day (kcal)
|
REE
last intubated day (kcal)
|
APACHEⅡ score
on ICU admission
|
CRP
1st intubated day (mg/dL)
|
CRP
2nd intubated day (mg/dL)
|
CRP
last intubated day (mg/dL)
|
SOFA
1st intubated day
|
SOFA
2nd intubated day
|
SOFA
last intubated day
|
RQ
1st intubated day
|
RQ
2nd intubated day
|
RQ
last intubated day
|
energy intake
1st intubated day (kcal)
|
energy intake
2nd intubated day (kcal)
|
energy intake
last intubated day (kcal)
|
sedatives
1st intubated day (mg/hr)
|
sedatives
2nd intubated day (mg/hr)
|
sedatives
last intubated day (mg/hr)
|
|
1
|
45
|
female
|
miliary tuberculosis
|
22
|
159
|
31
|
11.2
|
1027
|
1097
|
1239
|
1043
|
1263
|
32
|
21
|
18
|
1.1
|
13
|
10
|
8
|
0.69
|
0.78
|
0.74
|
500
|
690
|
1440
|
propofol 80 / fentanyl 0.05
|
propofol 100 / fentanyl 0.025
|
fentanyl 0.05
|
|
2
|
63
|
female
|
pneumonia
|
12
|
145
|
39
|
18.5
|
994
|
1012
|
1183
|
1137
|
1040
|
33
|
33.9
|
32
|
6.2
|
12
|
14
|
7
|
0.88
|
0.81
|
0.81
|
680
|
680
|
1250
|
propofol 50 / fentanyl 0.05
|
propofol 50 / fentanyl 0.05
|
fentanyl 0.05
|
|
3
|
76
|
male
|
pneumonia
|
20
|
178
|
70
|
18.8
|
1406
|
1407
|
1604
|
1544
|
1307
|
20
|
30.1
|
28
|
3
|
9
|
8
|
4
|
0.64
|
0.8
|
0.78
|
480
|
960
|
1520
|
dexmedetomidine 0.032 / fentanyl 0.05
|
dexmedetomidine 0.032 / fentanyl 0.075
|
fentanyl 0.05
|
|
4
|
60
|
male
|
necrotising fasciitis
|
22
|
174
|
69
|
22.4
|
1478
|
1395
|
1640
|
1762
|
1637
|
17
|
22
|
22.8
|
2
|
5
|
7
|
3
|
0.74
|
0.85
|
0.97
|
240
|
680
|
2080
|
propofol 100 / fentanyl 0.05
|
dexmedetomidine 0.024 / fentanyl 0.05
|
0
|
|
5
|
49
|
male
|
renal abcess
|
6
|
157
|
56
|
22.8
|
1292
|
1515
|
1682
|
1551
|
1424
|
22
|
11.2
|
12
|
5.9
|
14
|
14
|
3
|
0.73
|
0.74
|
0.81
|
540
|
970
|
1180
|
propofol 100
|
propofol 60
|
propofol 60
|
|
6
|
38
|
male
|
phlegmone
|
6
|
174
|
86
|
28.3
|
1864
|
1859
|
2268
|
2148
|
2240
|
22
|
25.5
|
23.3
|
9.4
|
9
|
8
|
4
|
0.72
|
0.76
|
0.69
|
560
|
560
|
1540
|
propofol 150 / fentanyl 0.05
|
propofol 150 / fentanyl 0.05
|
fentanyl 0.05
|
|
7
|
42
|
female
|
cholangitis
|
4
|
162
|
81
|
31
|
1530
|
1503
|
1696
|
1592
|
1480
|
31
|
13.8
|
9.3
|
6.2
|
16
|
14
|
11
|
0.69
|
0.71
|
0.77
|
400
|
1080
|
1080
|
0
|
0
|
0
|
|
8
|
42
|
male
|
gastrointestinal perforation
|
3
|
174
|
111
|
36.5
|
2176
|
2145
|
1894
|
1772
|
2175
|
26
|
9.4
|
15.9
|
28.7
|
9
|
4
|
2
|
0.78
|
0.85
|
0.75
|
390
|
760
|
760
|
propofol 100 / fentanyl 0.05
|
propofol 150 / fentanyl 0.05
|
propofol 150 / fentanyl 0.05
|
|
9
|
77
|
female
|
candidemia
|
16
|
138
|
38
|
20
|
906
|
1003
|
1225
|
1100
|
1297
|
32
|
11.8
|
9.2
|
6.1
|
15
|
12
|
8
|
0.77
|
0.95
|
0.73
|
840
|
840
|
1060
|
dexmedetomidine 0.024 / fentanyl 0.05
|
dexmedetomidine 0.024 / fentanyl 0.05
|
dexmedetomidine 0.032 / fentanyl 0.05
|
|
10
|
72
|
male
|
pneumonia
|
4
|
165
|
40
|
14.6
|
950
|
1056
|
1072
|
1144
|
1018
|
20
|
3.7
|
5.6
|
7.2
|
9
|
9
|
6
|
0.68
|
1
|
0.76
|
1760
|
1760
|
1790
|
propofol 50
|
propofol 50
|
propofol 60 / fentanyl 0.05
|
|
11
|
68
|
female
|
pseudomembranous colitis
|
20
|
150
|
35
|
15.6
|
941
|
976
|
1015
|
1142
|
1228
|
32
|
22.6
|
27
|
4
|
10
|
11
|
6
|
0.77
|
0.78
|
1.06
|
300
|
820
|
1640
|
0
|
0
|
0
|
|
12
|
21
|
female
|
phlegmone
|
14
|
140
|
35
|
17.9
|
1144
|
1005
|
902
|
1109
|
1194
|
13
|
26.2
|
13.9
|
3.1
|
7
|
7
|
4
|
0.63
|
0.86
|
0.81
|
580
|
1060
|
1580
|
propofol 120 / fentanyl 0.025
|
propofol 120 / fentanyl 0.05
|
propofol 120 / fentanyl 0.05
|
|
13
|
73
|
female
|
extremity gangrene
|
4
|
147
|
30
|
13.9
|
865
|
930
|
1132
|
1122
|
1158
|
22
|
1
|
2.3
|
1
|
8
|
7
|
4
|
0.72
|
0.8
|
0.76
|
630
|
630
|
630
|
0
|
0
|
0
|
|
14
|
69
|
male
|
cholangitis
|
3
|
158
|
64
|
25.8
|
1275
|
1340
|
994
|
1172
|
1195
|
23
|
3.1
|
3
|
7.5
|
10
|
8
|
2
|
0.68
|
0.74
|
0.79
|
520
|
520
|
400
|
propofol 50 / dexmedetomidine 0.02 / fentanyl 0.05
|
propofol 50 / dexmedetomidine 0.024 / fentanyl 0.075
|
dexmedetomidine 0.024 / fentanyl 0.075
|
|
15
|
55
|
female
|
necrotising fasciitis
|
8
|
165
|
65
|
23.9
|
1318
|
1373
|
1349
|
1248
|
1337
|
25
|
44.8
|
45.4
|
4.6
|
11
|
12
|
3
|
0.69
|
0.78
|
0.84
|
630
|
1230
|
1540
|
propofol 50 / dexmedetomidine 0.024 / fentanyl 0.075
|
propofol 50 / dexmedetomidine 0.024 / fentanyl 0.075
|
fentanyl 0.05
|
|
16
|
60
|
female
|
pneumonia
|
6
|
159
|
84
|
33.2
|
1468
|
1421
|
1813
|
1696
|
1852
|
17
|
2.9
|
25.1
|
8.1
|
17
|
15
|
12
|
0.86
|
0.73
|
0.72
|
200
|
680
|
1580
|
propofol 80 / dexmedetomidine 0.032 / fentanyl 0.075
|
propofol 80 / dexmedetomidine 0.4 / fentanyl 0.075
|
propofol 60 / dexmedetomidine 0.4 / fentanyl 0.05
|
|
17
|
82
|
male
|
pneumonia
|
25
|
152
|
47
|
20.3
|
916
|
1138
|
1158
|
1367
|
1161
|
22
|
28.7
|
27
|
2.1
|
12
|
13
|
9
|
0.72
|
0.68
|
0.73
|
870
|
870
|
1480
|
dexmedetomidine 0.024 / fentanyl 0.075
|
dexmedetomidine 0.024 / fentanyl 0.05
|
fentanyl 0.025
|
|
18
|
50
|
male
|
pelvic abscess
|
6
|
160
|
72
|
28.1
|
1523
|
1698
|
1634
|
1582
|
1692
|
25
|
16.5
|
20.8
|
20.1
|
19
|
19
|
14
|
1
|
0.7
|
0.74
|
430
|
620
|
940
|
propofol 120 / fentanyl 0.05
|
propofol 80 / fentanyl 0.05
|
propofol 120 / dexmedetomidine 0.32 / fentanyl 0.05
|
|
19
|
37
|
male
|
extremity gangrene
|
4
|
165
|
58
|
21.3
|
1443
|
1538
|
1665
|
1514
|
1535
|
25
|
25.2
|
25
|
22.1
|
16
|
15
|
12
|
0.79
|
0.85
|
0.81
|
200
|
1000
|
1240
|
dexmedetomidine 0.032 / fentanyl 0.05
|
dexmedetomidine 0.04 / fentanyl 0.05
|
dexmedetomidine 0.04 / fentanyl 0.05
|
|
20
|
37
|
male
|
pneumonia
|
3
|
172
|
51
|
17.2
|
1372
|
1457
|
1221
|
1081
|
1131
|
16
|
10.8
|
18.1
|
17.7
|
8
|
9
|
7
|
0.79
|
0.8
|
0.85
|
560
|
1240
|
1240
|
propofol 70
|
propofol 50 / midazolam 10
|
propofol 50 / midazolam 10
|
|
21
|
78
|
male
|
septic arthritis
|
7
|
152
|
69
|
29.9
|
1254
|
1395
|
1652
|
1294
|
1698
|
24
|
27.8
|
27.5
|
8.9
|
15
|
15
|
12
|
0.68
|
0.87
|
0.79
|
300
|
630
|
1240
|
dexmedetomidine 0.024 / fentanyl 0.05
|
dexmedetomidine 0.024 / fentanyl 0.05
|
dexmedetomidine 0.032 / fentanyl 0.1
|
|
22
|
63
|
male
|
pneumonia
|
3
|
174
|
54
|
17.8
|
1256
|
1220
|
1624
|
1586
|
1586
|
16
|
21.5
|
20.3
|
21
|
9
|
10
|
8
|
0.7
|
0.69
|
0.72
|
240
|
520
|
840
|
propofol 100 / fentanyl 0.05
|
propofol 50 / fentanyl 0.075
|
dexmedetomidine 0.02 / fentanyl 0.075
|
|
23
|
70
|
male
|
tetanus
|
23
|
164
|
57
|
21.2
|
1192
|
1255
|
1445
|
1519
|
1647
|
25
|
8.8
|
20.1
|
1.4
|
12
|
12
|
7
|
0.87
|
0.78
|
0.84
|
360
|
1080
|
1700
|
propofol 150 / fentanyl 0.05
|
propofol 100 / fentanyl 0.05
|
midazoram 10
|
|
24
|
53
|
male
|
pneumonia
|
4
|
164
|
61
|
22.7
|
1368
|
1572
|
1512
|
1417
|
1415
|
19
|
17.6
|
27.2
|
7.2
|
12
|
12
|
9
|
0.78
|
0.83
|
0.85
|
740
|
740
|
1340
|
propofol 100 / fentanyl 0.05
|
propofol 100 / fentanyl 0.025
|
propofol 100 / fentanyl 0.05
|
|
25
|
59
|
male
|
pneumonia
|
3
|
185
|
68
|
19.9
|
1529
|
1652
|
2134
|
2018
|
2185
|
15
|
4
|
11.3
|
7.4
|
9
|
9
|
3
|
0.71
|
0.7
|
0.75
|
300
|
300
|
300
|
dexmedetomidine 0.04 / fentanyl 0.05
|
dexmedetomidine 0.04 / fentanyl 0.05
|
dexmedetomidine 0.024 / fentanyl 0.05
|
|
26
|
82
|
male
|
pneumonia
|
4
|
163
|
57
|
21.5
|
1111
|
1255
|
1426
|
1413
|
1647
|
16
|
26.8
|
24.4
|
6.8
|
10
|
9
|
6
|
0.7
|
0.68
|
0.71
|
580
|
1060
|
1540
|
dexmedetomidine 0.024
|
dexmedetomidine 0.024
|
dexmedetomidine 0.024 / fentanyl 0.05
|
|
27
|
28
|
female
|
phlegmone
|
4
|
162
|
77
|
29.3
|
1554
|
1527
|
1384
|
1504
|
1517
|
35
|
2.5
|
9.7
|
6.9
|
13
|
11
|
8
|
0.71
|
0.77
|
0.81
|
400
|
640
|
880
|
fentanyl 0.1
|
dexmedetomidine 0.02 / fentanyl 0.05
|
dexmedetomidine 0.02 / fentanyl 0.05
|
|
28
|
74
|
male
|
pneumonia
|
3
|
163
|
56
|
22.2
|
1154
|
1243
|
1147
|
1079
|
1118
|
26
|
6
|
8.1
|
9.2
|
8
|
8
|
2
|
0.86
|
0.84
|
0.68
|
500
|
660
|
1380
|
propofol 80 / dexmedetomidine 0.02 / fentanyl 0.05
|
propofol 150 / dexmedetomidine 0.024 / fentanyl 0.05
|
propofol 150 / dexmedetomidine 0.02 / fentanyl 0.05
|
|
29
|
56
|
female
|
tetanus
|
13
|
150
|
52
|
23.1
|
1161
|
1268
|
1193
|
1080
|
1268
|
18
|
0.5
|
1.9
|
6.8
|
9
|
7
|
5
|
0.89
|
0.93
|
0.92
|
680
|
620
|
1600
|
propofol 80 / fentanyl 0.05
|
propofol 60 / fentanyl 0.05
|
fentanyl 0.05
|
|
30
|
80
|
male
|
pneumonia
|
3
|
150
|
44
|
19.6
|
876
|
1102
|
991
|
1014
|
978
|
18
|
8.3
|
6
|
7
|
8
|
7
|
3
|
0.75
|
0.82
|
0.76
|
200
|
200
|
400
|
dexmedetomidine 0.02
|
dexmedetomidine 0.028
|
dexmedetomidine 0.028
|
|
31
|
80
|
male
|
pneumonia
|
6
|
157
|
59
|
23.9
|
1122
|
1278
|
1221
|
1198
|
1268
|
20
|
1.1
|
3
|
4
|
10
|
9
|
8
|
0.76
|
0.72
|
0.8
|
420
|
420
|
840
|
propofol 50 / fentanyl 0.025
|
propofol 50 / fentanyl 0.025
|
0
|
|
32
|
73
|
male
|
pneumonia
|
9
|
162
|
81
|
30.9
|
1499
|
1536
|
1672
|
1531
|
1432
|
19
|
10.1
|
12.3
|
5.6
|
8
|
8
|
4
|
0.68
|
0.7
|
0.71
|
240
|
800
|
920
|
propofol 100 / fentanyl 0.05
|
propofol 50 / dexmedetomidine 0.032 / fentanyl 0.05
|
propofol 40 / dexmedetomidine 0.024 / fentanyl 0.05
|
|
33
|
85
|
male
|
gastrointestinal perforation
|
4
|
165
|
50
|
18.4
|
1004
|
1173
|
1213
|
1293
|
1197
|
34
|
15.3
|
19.3
|
24.5
|
13
|
12
|
9
|
0.79
|
0.75
|
0.82
|
380
|
480
|
1280
|
propofol 60 / fentanyl 0.05
|
propofol 100 / fentanyl 0.05
|
propofol 80 / fentanyl 0.05
|
|
34
|
66
|
female
|
ileus
|
18
|
151
|
40
|
17.5
|
1001
|
1021
|
1032
|
1003
|
1103
|
25
|
4.2
|
21
|
2.1
|
12
|
10
|
6
|
0.68
|
0.71
|
0.77
|
300
|
640
|
900
|
dexmedetomidine 0.024 / fentanyl 0.05
|
dexmedetomidine 0.032 / fentanyl 0.05
|
fentanyl 0.05
|
|
35
|
74
|
male
|
pneumonia
|
8
|
165
|
44
|
16.2
|
993
|
1102
|
1308
|
1238
|
1143
|
39
|
33.4
|
35.6
|
4.1
|
12
|
14
|
10
|
0.86
|
0.78
|
0.89
|
200
|
1120
|
1570
|
fentanyl 0.05
|
dexmedetomidine 0.04 / fentanyl 0.05
|
dexmedetomidine 0.02 / fentanyl 0.05
|
|
36
|
69
|
female
|
miliary tuberculosis
|
3
|
146
|
31
|
14.5
|
895
|
940
|
852
|
797
|
767
|
33
|
5.8
|
20.7
|
9.1
|
8
|
6
|
6
|
0.82
|
0.72
|
0.74
|
680
|
680
|
780
|
fentanyl 0.025
|
fentanyl 0.025
|
fentanyl 0.025
|
|
37
|
62
|
male
|
pneumonia
|
4
|
162
|
53
|
20.2
|
1223
|
1208
|
1107
|
1245
|
1112
|
20
|
2.2
|
1.5
|
0.7
|
6
|
8
|
5
|
0.89
|
0.91
|
0.86
|
400
|
960
|
1440
|
dexmedetomidine 0.024
|
dexmedetomidine 0.02 / fentanyl 0.05
|
dexmedetomidine 0.024
|
|
38
|
79
|
female
|
gastrointestinal perforation
|
3
|
150
|
50
|
22.1
|
1029
|
1112
|
1183
|
1221
|
1255
|
24
|
19.9
|
13
|
11.3
|
9
|
6
|
4
|
0.71
|
0.72
|
0.66
|
400
|
400
|
830
|
dexmedetomidine 0.032 / fentanyl 0.05
|
dexmedetomidine 0.04 / fentanyl 0.05
|
dexmedetomidine 0.032 / fentanyl 0.05
|
|
39
|
76
|
female
|
gastrointestinal perforation
|
4
|
152
|
62
|
22.2
|
1167
|
1221
|
1047
|
1031
|
1162
|
23
|
10
|
23.1
|
6.8
|
10
|
10
|
5
|
0.71
|
0.87
|
0.95
|
320
|
960
|
1480
|
propofol 50 / fentanyl 0.05
|
propofol 50 / fentanyl 0.05
|
fentanyl 0.025
|
|
40
|
61
|
male
|
pneumonia
|
3
|
156
|
67
|
27.5
|
1358
|
1372
|
1133
|
1231
|
1348
|
18
|
2.2
|
21.7
|
13.2
|
7
|
6
|
2
|
0.83
|
0.71
|
0.68
|
200
|
400
|
1440
|
dexmedetomidine 0.032 / fentanyl 0.05
|
dexmedetomidine 0.032 / fentanyl 0.05
|
dexmedetomidine 0.024
|
|
41
|
80
|
female
|
pneumonia
|
20
|
133
|
35
|
19.8
|
857
|
976
|
911
|
825
|
843
|
31
|
1.6
|
5.3
|
2.4
|
12
|
10
|
6
|
0.73
|
0.77
|
1.02
|
200
|
200
|
880
|
dexmedetomidine 0.02 / fentanyl 0.05
|
dexmedetomidine 0.02 / fentanyl 0.05
|
0
|
|
42
|
61
|
male
|
pneumonia
|
3
|
166
|
60
|
21.8
|
1303
|
1290
|
1365
|
1412
|
1241
|
26
|
7.6
|
16.3
|
18.1
|
10
|
9
|
5
|
0.79
|
0.73
|
0.75
|
320
|
320
|
320
|
propofol 50 / fentanyl 0.05
|
propofol 50 / fentanyl 0.05
|
propofol 50 / fentanyl 0.025
|
|
43
|
72
|
male
|
pneumonia
|
20
|
163
|
47
|
17.7
|
1036
|
1138
|
1174
|
1275
|
1239
|
33
|
5.8
|
8.7
|
7.4
|
13
|
12
|
8
|
0.97
|
0.75
|
0.78
|
200
|
780
|
1540
|
dexmedetomidine 0.02 / fentanyl 0.05
|
dexmedetomidine 0.024 / fentanyl 0.05
|
dexmedetomidine 0.02 / fentanyl 0.05
|
|
44
|
70
|
male
|
pneumonia
|
7
|
171
|
46
|
15.7
|
1066
|
1126
|
881
|
1001
|
1010
|
39
|
4.2
|
3.5
|
2.9
|
13
|
14
|
11
|
0.74
|
0.7
|
0.82
|
200
|
200
|
780
|
midazolam 5
|
midazoram 5
|
0
|
|
45
|
63
|
female
|
tetanus
|
31
|
153
|
54
|
23.1
|
1148
|
1148
|
1084
|
1362
|
1460
|
26
|
2.8
|
5
|
1
|
10
|
8
|
3
|
0.75
|
0.77
|
0.83
|
360
|
600
|
1120
|
propofol 150 / fentanyl 0.05
|
propofol 150 / fentanyl 0.05
|
0
|
|
46
|
77
|
male
|
pneumonia
|
6
|
155
|
65
|
27.1
|
1209
|
1348
|
1408
|
1427
|
1683
|
25
|
27.4
|
23.5
|
8.7
|
9
|
10
|
6
|
0.87
|
0.73
|
0.75
|
200
|
200
|
880
|
propofol 100 / fentanyl 0.05
|
propofol 150 / fentanyl 0.05
|
propofol 100 / dexmedetomidine 0.04 / fentanyl 0.05
|
|
47
|
56
|
female
|
gastrointestinal perforation
|
7
|
156
|
47
|
19.3
|
1126
|
1227
|
1205
|
1269
|
1321
|
35
|
24.8
|
28.7
|
12.8
|
14
|
11
|
8
|
0.73
|
0.7
|
0.87
|
400
|
600
|
840
|
dexmedetomidine 0.02 / fentanyl 0.05
|
dexmedetomidine 0.02 / fentanyl 0.05
|
fentanyl 0.025
|
Harris-Benedict equation (HBE) [4]
Males: BMR (kcal/day) = 66.5 + 13.8 × Weight (kg) + 5.0 × Height (cm) - 6.8 × age
Females: BMR (kcal/day) = 655.1 + 9.6 × Weight (kg) + 1.8 × Height (cm) - 4.7 × age.
Schofield equation [5]
Males: 18-30 years old: 15.057 × Weight (kg) + 692.2, 30-60 years old: 11.472 × Weight (kg) + 873.1, > 60 years old: 11.711 × Weight (kg) + 587.7
Females: 18-30 years old: 14.818 × Weight (kg) + 486.6, 30-60 years old: 8.126 × Weight (kg) + 845.6, > 60 years old: 9.082 × Weight (kg) + 658.5
We calculated total energy intake from the doctor’s order sheet. The decision regarding parenteral and/or enteral nutrition was made by a conference between the attending physician and ICU doctors. REE values measured by IC were not utilized in decision-making. Instead, for the subject with a state of good nutrition, we prescribed mainly enteral nutrients and the total energy intake was gradually increased over time. Whereas, for the subject with a state of poor nutrition, intravenous feeding solution was mainly administered.
Statistical analysis
Data analysis was performed using SigmaPlot 13 (Systat Software, Inc., San Jose, CA) and GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA). Quantitative variables were described as means and standard deviations. Differences between groups were compared using Mann-Whitney U test, paired t-test, one-way ANOVA, or two-way ANOVA post hoc test. P < 0.05 was considered significant.
Results
A total of 47 patients with sepsis were included in this study. Their demographic data is shown in Table 1. The patients who survived to discharge only were included, because most moribund patients showed unexpected values of respiratory quotient, within the range adopted as exclusion criteria. We compared REE measured by IC with predicted energy expenditure (as BMR) calculated by the equations mentioned above. As a stress factor, we adopted 1.4-fold rescaling for comparison between measured and predicted energy expenditure, because it has been reported that values between 1.2 and 1.6 should be used as the stress factor for septic patients. In the intubated patients in this study, REE was always smaller than the total energy expenditure (TEE) estimated by 1.4-fold rescaling of the HBE-based BMR (two-way ANOVA with post hoc Bonferroni test, P < 0.0001), as shown in Figure 1. This was also the case for BMR estimated by the Schofield equation (Figure 1). Since the TEE calculated by Harris-benedict and Schofield equations were not different, we used HBE for further analysis. REE values were plotted against BMR calculated by HBE to determine the stress factor for septic patients (Figure 2). REE and BMR correlated reasonably well on the first day (R = 0.79). Additionally, the slope of the graph, namely, REE/BMR, was 1.09. REE also correlated well with BMR on both the second and last days (R = 0.76 and 0.78, respectively). Surprisingly, REE/BMR on the second and last days were 1.07 and 1.11, respectively, suggesting that the estimated stress factor did not change over time (see also Table 2). Many patients in this study were administered some sort of sedative (Table 3, Table S1). However, REE/BMR did not depend on the number of sedatives administered to the patients throughout the intubation period (one-way ANOVA, P > 0.05). These results suggest that sedatives had minimal effects on REE/BMR.
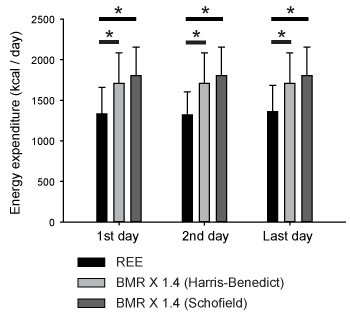
Figure 1. Comparison of the energy expenditure measured by indirect calorimetry (IC) and calculated by Harris-Benedict and Schofield equations. Basal metabolic rate (BMR) was multiplied by 1.4, as a tentative stress factor. Resting energy expenditure (REE) measured by IC was always smaller than estimated total energy expenditure by the prediction equation (two-way ANOVA with post hoc Bonferroni test, P < 0.0001).
Number of patients |
47 |
Male |
29 (61.7%) |
Age (years) |
63 ± 15 (21-85) |
Height (cm) |
159 ± 11 (133-185) |
Weight (kg) |
56 ± 17 (30-111) |
BMI (kg / m²) |
21.8 ± 5.4 (11-37) |
APACHEⅡ score |
25 ± 7 (13-39) |
BMR on admission:
Harris-Benedict (kcal/day) |
1221 ± 268 (857-2176) |
BMR on admission:
Schofiled (kcal/day) |
1289 ± 251 (930-2145) |
Primary site of infection |
|
respiratory |
24 |
skin and joint |
11 |
abdominal |
9 |
blood stream |
1 |
urinary |
2 |
Table 1. Patients’ demographic data.
Next, we sought the reasons for the relative constancy of REE/BMR in septic patients during the intubation period. To evaluate the severity of illness, sequential organ failure assessment (SOFA) scores and blood concentrations of C-reactive protein (CRP) were analyzed (Table 2). SOFA scores on the last day were lower than those on the first day (Mann-Whitney U test, P < 0.001). This trend corroborates with a decrease in CRP concentrations, suggesting improvement in the general condition of the patients. We also compared total energy intake (Table 2), since that may have impacted REE, and found that total energy intake on the last day was greater than that on the first day (Mann-Whitney U test, P < 0.001). Finally, we analyzed RQ and found that RQ on the last day was greater than that on the first day (Mann-Whitney U test, P = 0.04). Increased energy intake accompanied with RQ gain suggested that the exogenous energy supplied on the last intubation day was sufficient for the patients.
Discussion
In this study, we found that REE/BMR, i.e., the estimated stress factor, in survivors of sepsis was in the range of 1.07 to 1.11. Moreover, the estimated stress factor did not change over time during the intubation period (Figure 2 and Table 2).
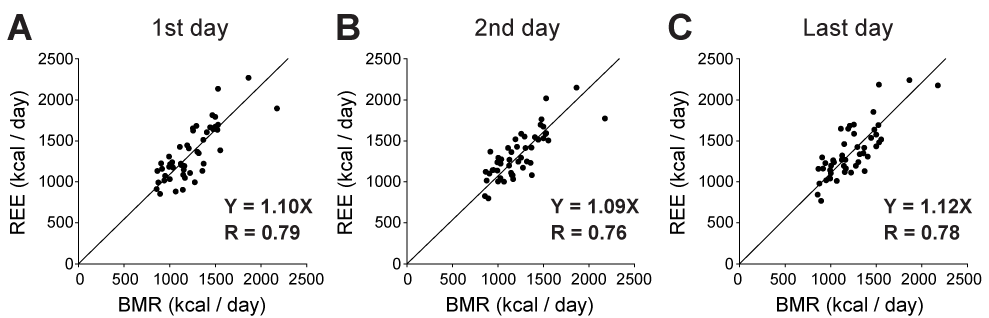
Figure 2. Relationship between resting energy expenditure (REE) and basal metabolic rate (BMR). REE and BMR correlated well on the first, second and last intubation days (R = 0.79. 0.76, and 0.78). The value of REE/BMR was always approximately 1.1.
|
1st day |
Last day |
P |
REE / BMR |
1.10 ± 0.16 |
1.12 ± 0.17 |
0.46 |
SOFA score |
10.9 ± 3.0 |
6.3 ± 3.1 |
< 0.001 |
CRP (mg / dL) |
14.2 ± 11.2 |
8.2 ± 6.6 |
0.02 |
Energy intake (kcal / day) |
448 ± 267 |
1171 ± 415 |
< 0.001 |
Respiratory quotient |
0.764 ± 0.08 |
0.797 ± 0.09 |
0.04 |
Table 2. Comparisons of the ratio of measured resting energy expenditure (REE) to basal metabolic rate (BMR), SOFA score, blood concentration of CRP, energy intake, and respiratory quotient between the first and last intubation days. Paired t-test was performed for comparison of REE/BMR. Mann-Whitney U test was used for analyses of the remaining data.
The numerical value of the stress factor that should be multiplied by BMR to predict total energy expenditure in critically ill patients remains controversial. Notably, the optimal number for septic patients is unknown. A previous study that included 73 mechanically ventilated, non-surgical, critically ill patients reported that the measured energy expenditure, namely REE, was no different from that predicted by the HBE, which does not involve multiplication by a stress factor [17]. The authors found that patients with sepsis are an exception to this and concluded that REE was ∼1.2 fold higher than that calculated by the unmodified formula during sepsis. Our estimated stress factor in septic patients was relatively small, but did not greatly differ from their data. One possible explanation for the small stress factor was sedation of the subjects. Indeed, it has been reported that REE/BMR decreased significantly by increasing the depth of sedation in postoperative patients [29]. In our study, all patients were intubated and most of them were sedated. However, REE/BMR was not found to be dependent on the number of sedatives administered (Table 3). Besides, patients who did not receive any sedatives might also have been drowsy due to endogenous sedative molecules, such as cannabinoids and ammonia. In other words, the degree of sedation might have been uniform regardless of whether or not sedatives were administered.
|
Number of sedatives used |
|
|
0 |
1 |
2 ≤ |
P |
1st day |
1.17 ± 0.13 (3) |
1.06 ± 0.19 (10) |
1.10 ± 0.15 (34) |
0.60 |
2nd day |
1.18 ± 0.13 (3) |
1.11 ± 0.16 (6) |
1.08 ± 0.15 (38) |
0.52 |
last day |
1.13 ± 0.16 (8) |
1.09 ± 0.14 (16) |
1.14 ± 0.19 (23) |
0.59 |
2021 Copyright OAT. All rights reserv
Table 3. Comparison of the ratios of measured resting energy expenditure (REE) to basal metabolic rate (BMR) on the three days in terms of the number of sedatives used. One-way ANOVA was used for analysis.
The lack of change in REE/BMR over time was likely caused by following mechanisms. It is known that greater energy intake results in an increase in REE. This was likely the case in this study. The increase in RQ on the last day suggested a shift of the main energy substrate from fats to carbohydrates (Table 2). This shift was also probably caused by the increased energy intake. Conversely, overfeeding on the last day may have resulted in enhanced glycogen and fat synthesis [30]. This is called “nutritional stress”. It has been reported that in the process of glycogen and fat synthesis, 5 and 20%, respectively, of the generated molecules are themselves used up during the process of synthesis [31]. Besides, consumption of glycogen and fat results in an increase in REE. Taken together, excessive energy intake on the last day likely resulted in an increase in REE accompanied by glycogen and fat synthesis. Whereas, decrease in stress hormones, including adrenaline, noradrenaline, cortisol, growth factor and glucagon, resulting from the improvement in the patients’ condition, probably caused a decrease in REE. Stress hormones are released from the adrenal gland or locus ceruleus in the early stages of sepsis, and these induce catabolism of skeletal muscle and fat. Although few studies have shown the effect of stress hormones on energy expenditure, research has shown that exogenous adrenaline and cortisol raise the metabolic rate [32,33]. On the last intubation day, the general condition of the patients in this study had improved significantly, as indicated by the decrease in serum levels of the inflammatory marker, CRP (Table 2). Therefore, we opined that resolution of the illness resulted in a decrease in stress hormones, which in turn led to the decrease in REE. Collectively, an increase in REE caused by the increased energy intake may have been counteracted by a decrease in REE caused by the improvement in the patients’ condition.
Our study has some limitations. This study did not have a specific protocol for nutrition control while the patients were intubated. Therefore, there may have been inter-individual differences in the types of nutrients that were administered to the patients. In theory, measured REE might fluctuate with this uncontrolled factor, because RQ depends on the class of nutrient consumed. To overcome this issue, prospective studies including patients under uniform nutrition control should be performed. Another possible limitation of this study is a small number of measurements per day. Although IC can continuously monitor REE, we adopted data measured at a single time point per day. This protocol was formulated to avert inconstancy in the patient’s condition that can affect REE measurement. Since we did not intend to utilize the data obtained from IC for nutrition control, slight overfeeding probably occurred in the late intubation period. Despite this, however, we believe that our findings regarding the stress factor in mechanically ventilated septic patients under sedation may contribute to estimation of total energy expenditure using prediction equations.
Conclusions
The measured REE by IC in septic patients under sedation was approximately 1.1 times greater than BMR. The ratio of measured REE to BMR does not change with resolution of the illness throughout the intubation period. This result was likely caused by the concomitant increase in energy intake and improvement in patient general condition at this time. These findings may contribute to better nutrition control in septic patients in the ICU.
Acknowledgements
The authors are grateful to all staff of the Intensive Care Unit of Gunma University Hospital for their continuous help and support.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All the authors participated in data collection. JK designed and conducted the study. JK and TT wrote the manuscript. FK and SS supervised the study. All the authors read and approved the final manuscript.
Funding
This study was supported by JSPS KAKENHI Grant Numbers 25893031 and 16K20376.
References
- Garrow JS (1976) Underfeeding and overfeeding and their clinical consequences. Proc Nutr Soc 35: 363-368. [Crossref]
- McClave SA, Lowen CC, Kleber MJ, Nicholson JF, Jimmerson SC, et al. (1998) Are patients fed appropriately according to their caloric requirements? JPEN J Parenter Enteral Nutr 22: 375-381. [Crossref]
- Schetz M, Casaer MP, Van den Berghe G (2013) Does artificial nutrition improve outcome of critical illness? Crit Care 17: 302. [Crossref]
- Harris JA, Benedict FG (1919) A biometric study of basal metabolism in man. Washington,: Carnegie Institution of Washington; 1919. vi, 266 p. incl. tables. p.
- Schofield WN (1985) Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr 39 Suppl 1: 5-41. [Crossref]
- Ireton-Jones CS, Turner WW Jr., Liepa GU, Baxter CR (1992) Equations for the estimation of energy expenditures in patients with burns with special reference to ventilatory status. J Burn Care Rehabil 13: 330-333. [Crossref]
- Frankenfield D, Smith JS, Cooney RN (2004) Validation of 2 approaches to predicting resting metabolic rate in critically ill patients. JPEN J Parenter Enteral Nutr 28: 259-264. [Crossref]
- Swinamer DL, Grace MG, Hamilton SM, Jones RL, Roberts P, et al. (1990) Predictive equation for assessing energy expenditure in mechanically ventilated critically ill patients. Crit Care Med 18: 657-661. [Crossref]
- Heyland DK, Dhaliwal R, Drover JW, Gramlich L, Dodek P, et al. (2003) Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN J Parenter Enteral Nutr 27: 355-373. [Crossref]
- Long CL, Schaffel N, Geiger JW, Schiller WR, Blakemore WS (1979) Metabolic response to injury and illness: estimation of energy and protein needs from indirect calorimetry and nitrogen balance. JPEN J Parenter Enteral Nutr 3: 452-456. [Crossref]
- Brandi LS, Santini L, Bertolini R, Malacarne P, Casagli S, et al. (1999) Energy expenditure and severity of injury and illness indices in multiple trauma patients. Crit Care Med 27: 2684-2689. [Crossref]
- Alexander E, Susla GM, Burstein AH, Brown DT, Ognibene FP (2004) Retrospective evaluation of commonly used equations to predict energy expenditure in mechanically ventilated, critically ill patients. Pharmacotherapy 24: 1659-1667. [Crossref]
- MacDonald A, Hildebrandt L (2003) Comparison of formulaic equations to determine energy expenditure in the critically ill patient. Nutrition 19: 233-239. [Crossref]
- Casati A, Colombo S, Leggieri C, Muttini S, Capocasa T, et al. (1996) Measured versus calculated energy expenditure in pressure support ventilated ICU patients. Minerva Anestesiol 62: 165-170. [Crossref]
- O'Leary-Kelley CM, Puntillo KA, Barr J, Stotts N, Douglas MK (2005) Nutritional adequacy in patients receiving mechanical ventilation who are fed enterally. Am J Crit Care 14: 222-231. [Crossref]
- Wu C, Wang X, Yu W, Tian F, Liu S, et al. (2015) Hypermetabolism in the Initial Phase of Intensive Care Is Related to a Poor Outcome in Severe Sepsis Patients. Ann Nutr Metab 66: 188-195. [Crossref]
- Liggett SB, Renfro AD (1990) Energy expenditures of mechanically ventilated nonsurgical patients. Chest 98: 682-686. [Crossref]
- Losser MR, Damoisel C, Payen D (2010) Bench-to-bedside review: Glucose and stress conditions in the intensive care unit. Crit Care 14: 231. [Crossref]
- Soares MJ, Sheela ML, Kurpad AV, Kulkarni RN, Shetty PS (1989) The influence of different methods on basal metabolic rate measurements in human subjects. Am J Clin Nutr 50: 731-736. [Crossref]
- Coss-Bu JA, Jefferson LS, Walding D, David Y, Smith EO, et al. (1998) Resting energy expenditure and nitrogen balance in critically ill pediatric patients on mechanical ventilation. Nutrition 14: 649-652. [Crossref]
- WEIR JB (1949) New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109: 1-9. [Crossref]
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, et al. (2003) 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 31: 1250-1256. [Crossref]
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, et al. (2013) Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41: 580-637. [Crossref]
- Kross EK, Sena M, Schmidt K, Stapleton RD (2012) A comparison of predictive equations of energy expenditure and measured energy expenditure in critically ill patients. J Crit Care 27: 321 e5-12. [Crossref]
- Wooley JA (2006) Indirect calorimetry: applications in practice. Respir Care Clin N Am 12: 619-633. [Crossref]
- McClave SA, Martindale RG, Kiraly L (2013) The use of indirect calorimetry in the intensive care unit. Curr Opin Clin Nutr Metab Care 16: 202-208. [Crossref]
- Schlein KM, Coulter SP (2014) Best practices for determining resting energy expenditure in critically ill adults. Nutr Clin Pract 29: 44-55. [Crossref]
- Matarese LE (1997) Indirect calorimetry: technical aspects. J Am Diet Assoc 97: S154-160. [Crossref]
- Terao Y, Miura K, Saito M, Sekino M, Fukusaki M, et al. (2003) Quantitative analysis of the relationship between sedation and resting energy expenditure in postoperative patients. Crit Care Med 31: 830-833. [Crossref]
- Flatt JP (1970) Conversion of carbohydrate to fat in adipose tissue: an energy-yielding and, therefore, self-limiting process. J Lipid Res 11: 131-143. [Crossref]
- Shimada T (1994) Metabolism under total parenteral nutrition: the influence of different compositions of energy substrate. Nihon Geka Gakkai Zasshi 95: 295-305. [Crossref]
- Brillon DJ, Zheng B, Campbell RG, Matthews DE (1995) Effect of cortisol on energy expenditure and amino acid metabolism in humans. Am J Physiol 268: E501-513. [Crossref]
- Ratheiser KM, Brillon DJ, Campbell RG, Matthews DE (1998) Epinephrine produces a prolonged elevation in metabolic rate in humans. Am J Clin Nutr 68: 1046-1052. [Crossref]


