Abstract
Sheep is a species that shows 85% overall identity of the adenosine A3 receptor with its human ortholog. In this study we demonstrate a role for the adenosine A3 receptor in ovine allergic airway responses. In isolated tracheal preparations taken from sheep naturally sensitive to Ascaris suum, antigen challenge evoked airway smooth muscle contraction. The smooth muscle contractile response was dependent on the applied Ascaris suum antigen dose. The response to a threshold dose of antigen was enhanced in the presence of an adenosine A3 agonist AB-MECA (10-6M), suggesting that adenosine A3 receptor function contributed to the enhanced allergen response. This was confirmed by showing that a selective adenosine A3 receptor antagonist SSR16142, inhibited the increased antigen response with EC50=2·10-9M. We then tested the effects of SSR161421, given intravenously, on antigen-induced responses in allergic sheep following inhalation of Ascaris suum, in-vivo. SSR161421 (0.3-3 mg/kg) provided dose-dependent inhibition of the antigen-induced late bronchoconstrictor responses and the post antigen-induced airway hyper responsiveness. This is the first demonstration that a selective adenosine A3 receptor antagonist inhibits antigen-induced responses in a species with an A3 receptor that bears significant similarity to its human counterpart.
Key words
adenosine A3, sheep, Ascaris suum, bronchoconstriction, early and late allergic response, SSRl61421
Introduction
Adenosine is thought to be an important pathological mediator in asthma. Adenosine evokes bronchoconstriction in airways by directly acting in bronchial smooth muscle cells or indirectly by inducing the release of mast cell mediators and by acting on airway afferent nerve endings [1-4].
Allergen challenge increases the bronchial level of adenosine [5], which can affect mast cell function [6]. Exogenous adenosine induces and/or enhances antigen-induced mast cell degranulation through the activation of A2b and/or A3 adenosine receptors [7-9]. Inhaled adenosine causes bronchoconstriction in asthmatic patients but not healthy subjects [4,10].
The potential role of adenosine A3 receptor stimulation in the pathophysiology of asthma, led to the development of a novel adenosine A3 receptor selective antagonist SSR161421. SSR161421 is a nanomolar adenosine A3 receptor antagonist with at least three log selectivity towards other adenosine receptors (A1, A2a, A2b) and adenosine unrelated molecular targets [11]. We recently showed that SSR161421 has significant in-vivo pharmacologic activity against adenosine A3 ligand specific and allergic disease models in rodents and guinea pigs [11,12]. However, mouse and guinea pig adenosine A3 receptors shows only 73% and 75 % identity with human adenosine A3 receptor sequence, respectively.
To extend our studies and further identify a role for A3 receptor stimulation in asthma, we thought to determine if the effectiveness of SSR161421 in a species where the adenosine A3 receptor sequence is closer to that found in man. For this purpose we chose to use sheep because the ovine adenosine A3 receptor shows higher i.e., 85% overall identity with human A3 receptor [13]. Use of the sheep model of experimental asthma also allowed us to test the activity of SSR161421 on antigen-induced early and late responses and post antigen-induced airway hyperresponsiveness, end points not accessible in the previous studies.
Materials and methods
Isolated tracheal preparation
The animals were tissue donors therefore, the approval of the experimental protocol by the Hungarian Governmental Animal Ethics Committee (ÁTET) was not mandatory. However, all experiments were performed in accordance with the Institutional Ethical Codex, Hungarian Act of Animal Care and Experimentation (1998, XXVIII, section 243/1998) and the European Union guidelines (directive 2010/63/EU. A modified method of Abraham et al., [14] was used. Male Merino sheep (35-50 kg b.w) were used for the experiments. Sheep were fed conventional sheep food (hay). The animals were sacrificed by i.v. overdose of pentobarbital and the trachea was removed and placed immediately in Krebs-Henseleit solution of the following composition (mM): NaCI-1I8, KCI-4.7, CaC12·H2O-2.5, KH2PO4-1.2, NaHC03-25.0, MgSO4·7H2O-1.2 and glucose 11.1. The trachea was carefully stripped of connective tissue and one cartilaginous wide ring was cut. These rings were opened longitudinally, the cartilage was removed, smooth muscles containing part mounted into an organ bath containing Krebs-Henseleit solution, maintained at 39°C and were continuously bubbled with 95% O2 and 5% CO2 to obtain pH of 7.4 ± 0.1. The preparations were preloaded with 2.0 g tension. The tissue samples then were allowed to equilibrate for 1.5-2 hours, during which time they were washed approximately every 20 min with fresh Krebs-Henseleit solution. Having developed a stable tracheal tone, 4·10-6 M acetylcholine (ACh) was added to the organ bath twice interrupted and followed by wash out periods. After the preparation reestablished a normal baseline tone, they were treated with DMSO (vehicle of SSR161421) or SSRl61421 and 20 min later with saline (vehicle of AB-MECA) or 10-6 M AB-MECA and finally the tracheal contractions were evoked with Ascaris suum antigen. Since the tracheal preparations showed varied sensitivity to Ascaris suum antigen, the applied antigen doses varied in the 0.1 Unit/mL-100 Unit/mL range to evoke 13.9 ± 2.9% (1.4 ± 1.2 g) tracheal contractions in AB-MECA free control groups. For statistical analyses One-way analysis of variance followed by Dunnett's test was used.
Conscious sheep
All procedures were approved by the Mount Sinai Medical Center Animal Research Committee, which is responsible for assuring the humane care and use of experimental animals. All animals used in this study were allergic to Ascaris Suum antigen and had previously demonstrated early and late airway responses, and post antigen-induced airway hyperresponsiveness to inhaled carbachol, following inhalation challenge with Ascaris suum antigen [15].
Measurement of airway mechanics
The unsedated sheep were restrained in a cart in the prone position with their heads immobilized. After topical anesthesia of the nasal passages with 2% lidocaine solution, a balloon catheter was advanced through one nostril into the lower esophagus. The animals were intubated with a cuffed endotracheal tube through the other nostril using a flexible fiberoptic bronchoscope as a guide. Pleural pressure was estimated with the esophageal balloon catheter. Once the balloon was placed, it was secured so that it remained in position for the duration of the experiment. Lateral pressure in the trachea was measured with a sidehole catheter (inner dimension, 2.5 mm) advanced through and positioned distal to the tip of the endotracheal tube. Transpulmonary pressure, the difference between tracheal and pleural pressure, was measured with a differential pressure transducer catheter system. For the measurement of pulmonary resistance (RL, the proximal end of the endotracheal tube was connected to a pneumotachograph. The signals of flow and transpulmonary pressure were recorded on an oscilloscope recorder which was linked to a computer for on-line calculation of RL from transpulmonary pressure, respiratory volume (obtained by digital integration) and flow. Analysis of 5-10 breaths were used for the determination of RL in cm H20 /L·sec-1.
Aerosol delivery systerms
Aerosols of Ascaris suum extract (diluted 20:1 with phosphate buffered saline; 82,000 PNU/ml) were generated using a disposable medical nebulizer (RaindropR, Puritan Bennett), which produces an aerosol with a mass median aerodynamic diameter of 3.2 µm (geometric standard deviation, 1.9) as determined by a 7 stage Andersen cascade impactor. The output from the nebulizer was directed into a plastic t-piece, one end of which was connected to the inspiratory port of a Harvard respirator. To better control aerosol delivery, a dosimeter consisting of a solenoid valve and a source of compressed air (20 psi) was activated at the beginning of the inspiratory cycle of the Harvard respirator system for 1 s. The aerosol was delivered at a tidal volume of 500 ml and a rate of 20 breaths per minute for 20 minutes. Each sheep was challenged with an equivalent dose of antigen (400 breaths) in the control and drug trials. Carbachol aerosols were also generated with this same nebulizer system for the carbachol dose response curves. Measurements of RL were repeated immediately after inhalation of buffer and after each administration of 10 breaths of increasing concentrations of carbachol solution (0.25%, 0.5%, 1.0%, 2.0% and 4.0% w/v). To assess airway responsiveness, the cumulative carbachol dose in breath units (BU) that increased RL 400% over the post-buffer value (i.e., PC 400) was calculated from the dose response curve. One breath unit was defined as one breath of a 1% w/v carbachol solution.
Drug treatments
Three doses of SSR161421 were used: 3 mg/kg (n=4), 1 mg/kg (n=4), and 0.3 mg/kg (n=4). The drug was dissolved in PEG-400, 1-methyl pyrrolidinone, distilled water: (6:2:2) and was given i.v. 10 min before antigen challenge.
Experimental protocol
The protocol used for this study was to first obtain baseline dose response curves to aerosol carbachol 1-3 days before antigen challenge. Then on the day of antigen challenge, the sheep received either 3 mg/kg, 1 mg/kg or 0.3 mg/kg SSR161421 by i.v. injections 10 min before antigen challenge. On the challenge day values of lung resistance (RL) were measured at baseline and, then, after drug/placebo treatment. The animals were, then, challenged with Ascaris suum antigen and RL was remeasured immediately after challenge, hourly from l-6 h after challenge and on the half-hour from 6.5 h to 8 h after challenge. Measurements of RL were obtained 24 h after challenge followed by the 24 h post challenge dose response curve. Responses to antigen challenge with drug treatment were compared to each animal's historical control data. A two-tailed paired t-test was used to assess pairwise differences between historical control and drug treatment. The variables assessed were the peak early airway response (maximum increase in RL, 0-4 h after challenge), mean late airway response (average increase in RL between 5-8h after antigen challenge,) and on the ratio of post challenge PC400 to pre challenge PC400. Values in the text and Figure 1 and 2 are mean ± SE for 4 sheep. Statistical analysis of these variables is provided in Table 1.
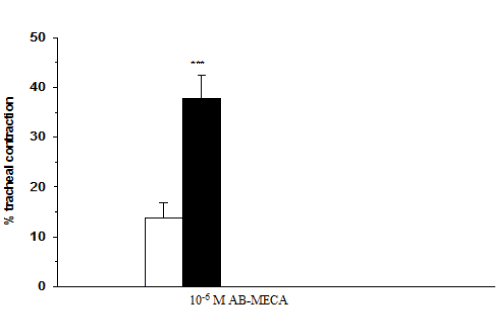
Figure 1. Ascaris suum antigen-induced tracheal contraction without (white column) and with (black column) 10-6 M AB-MECA (mean ± SEM, n=16-28, ***p<0.001, Student's t test.)
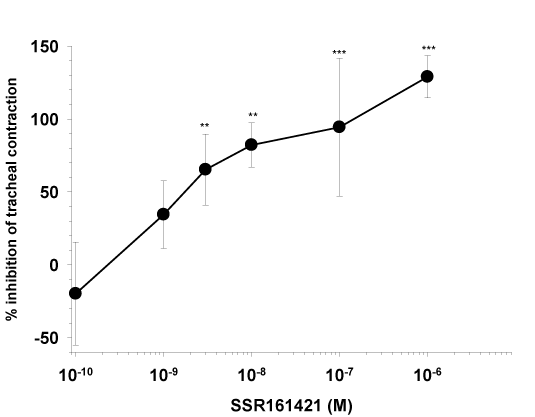
Figure 2. Effect of SSR161421 on 10-6 M AB-MECA enhanced Ascaris suum antigen-induced tracheal contraction (mean ± SEM, n=9-12, **p<0.01, ***p<0.001, One-Way Analysis of Variance followed by Dunnett's test
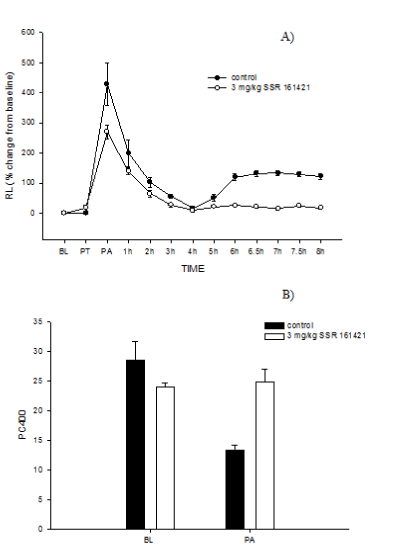
Figure 3. A) Time course of antigen-induced changes in lung resistance (RL) in four sheep treated with 3 mg/kg SSR 161421 i.v., 10 min before antigen challenge. Responses are compared to the animals’ historical control. BL=baseline, PT=post treatment, PA=post antigen challenge. B) Effect of 3 mg/kg SSR 161421 on airway responsiveness. A decrease in the PC 400 indicates the development of airway hyperresponsiveness. Values are mean ± se for 4 sheep. Statistical analysis is found in Table 1. BL baseline PA=24h post antigen.
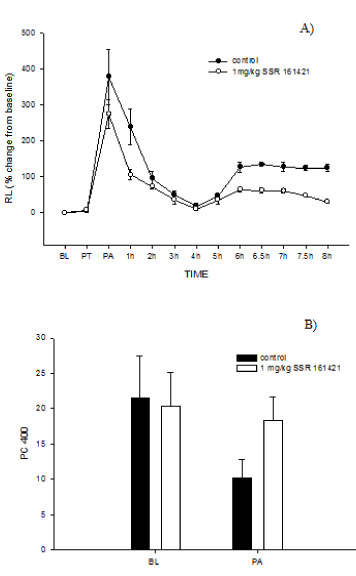
Figure 4. A) Time course of antigen-induced changes in lung resistance (RL) in four sheep treated with 1 mg/kg SSR 161421 i.v., 10 min before antigen challenge. Responses are compared to the animals’ historical control. BL=baseline, PT= post treatment, PA = post antigen challenge. B) Effect of 1 mg/kg SSR 161421 on airway responsiveness. A decrease in the PC 400 indicates the development of airway hyperresponsiveness. Values are mean ± se for 4 sheep. Statistical analysis is found in Table 1. BL baseline PA=24h post antigen.
Treatment |
EARa,b |
LARa,b |
AHRa,c |
Control |
428 ± 71 % |
114 ± 13 % |
0.47 ± 0.03 |
SSR 161421 (3 mg/kg) |
270 ± 24 %e |
21 ± 1 %d |
1.03 ± 0.07d |
Control |
377 ± 77 % |
114 ± 13% |
0.48 ± 0.03 |
SSR 161421 (1 mg/kg) |
274 ± 39% |
49 ± 6 %d |
0.95 ± 0.11d |
Control |
570 ± 58% |
110 ± 12% |
0.46 ± 0.02 |
SSR 161421 (0.3 mg/kg) |
603 ± 26% |
103 ± 11% |
0.44 ± 0.06 |
aAll results are presented as mean ± SE for n=4. bValues for peak EAR (largest value of lung resistance for each sheep between 0-4h) and mean LAR(average value of lung resistance for each sheep between 5-8h are presented as percentage change in specific lung resistance from baseline. cValues for AHR are post challenge PC400: Pre-challenge PC400 ratio. A value close to 1 indicates that there is no change in airway responsiveness. Values less than 1 indicate the development of AHR. Paired t-Testd P<0.05 vs Control; eP<0.10 vs. Control. |
Table 1: Effects of SSR161421 on Antigen-Induced Responses
Materials
AB-MECA HCI salt (N6-(4-aminobenzyl)-adenosine-5'-N-methyl-uronamide dihydrochloride) and SSRl61421 (4-methoxy-N-(benylamino-3-cyano-quinolin-2-yl)-benzamide was synthetized in Chinoin Co. Ltd. as described (WO02096879). Ascaris suum antigen was purchased from Greer Diagnostics, Lenoir, NC. All the other materials were obtained from Sigma-Aldrich Co.
Results
Isolated tracheal preparations
2021 Copyright OAT. All rights reserv
Due to the high variability of the antigen sensitivity of the animals 3·10-6 Unit/mL-100 Unit/mL range of Ascaris suum antigen was used to evoke threshold (11.5 ± 2.3%) tracheal contractions. The adenosine A3 receptor agonist AB-MECA (10-6 M), significantly increased Ascaris suum antigen induced tracheal contractions from 11.5 ± 2.3% (1.4 ± 1.2 g) to 36.7 ± 4.0% (4.5 ± 1.7g) (Figure 1). This AB-MECA enhanced tracheal contraction was inhibited by SSR161421 (EC50=2·10-9 M) as illustrated in Figure 2. The highest concentration of SSR161421 (10-6 M) showed 129 ± 15% inhibition, indicating that at this concentration, SSR161421 not only completely reversed the AB-MECA enhanced contraction, but also inhibited the antigen evoked response as well.
Conscious sheep
SSR161421 at both 3 mg/kg and 1 mg/kg (Figure 3 and 4) provided significant protection against the mean late airway response (LAR) and the post antigen-induced airway hyperresponsiveness (AHR) Table 1. There was a slight effect of 3 mg/kg SSR161421 on the early airway response (EAR: P=0.087), but this effect did not achieve statistical significance. At 0.3 mg/kg, SSR161421 had no effect on any measured parameter, thus identifying the no-effect dose (Figure 5).

Figure 5. A) Time course of antigen-induced changes in lung resistance (RL) in four sheep treated with 0.3 mg/kg SSR161421 i.v., 10 min before antigen challenge. Responses are compared to the animals’ historical control. BL=baseline, PT= post treatment, PA=post antigen challenge. B) Effect of 0.3 mg/kg SSR 161421 on airway responsiveness. A decrease in the PC 400 indicates the development of airway hyperresponsiveness. Values are mean ± se for 4 sheep. Statistical analysis is found in Table 1. BL baseline PA=24h post antigen.
Discussion
Adenosine induces bronchoconstriction through multiple mechanisms. In asthmatic subjects the effect of adenosine is sensitive to muscarinic receptor antagonists, suggesting that adenosine mediates obstruction at least partly indirectly [16-18]. This observation was also consistent with the preclinical evidence that adenosine can activate afferent nerves in-vivo [1,2]. However, atropine as a muscarinic agonist didn’t abolish completely the bronchoconstriction in respose to adenosine. This residual, muscarinic insensitive component of the response is mediated by direct activation airway smooth muscle [19-21] and/or indirectly via mediators released from other cell types expressing these receptors. It is hypothesized that A2b in humans, but A3 receptor in rodents, would mediate, indirectly, the bronchoconstriction in response to adenosine [21]. On the other hand, A1 receptor over-expressed on asthmatic airways would mediate a direct adenosine bronchoconstrictor effect [19,21]. Also on human bronchial tissue contracted in a concentration-dependent manner to adenosine agonists that showed a rank order of activity of A1>A2b>>A2a & A3 [22].
The pulmonary levels of endogenous adenosine can be elevated in different disease such as asthma and COPD [5]. Patients with these diseases show higher pulmonary adenosine levels and also react to adenosine inhalation with bronchoconstriction [4,10]. This is not the case in healthy subjects, suggesting that adenosine may be an important mediator in diseased airways.
Adenosine is an endogenous nucleotide that can regulate mast cells function [6]. Adenosine acting on mast cell surface A2b and/or A3 receptors evokes degranulation and/or enhances the antigen -induced release of mast cell mediators [7-9]. Currently, there is a controversy as to whether the A2b or A3 receptor has a more dominant role in the pathophysiology of asthma. To gain insight into this problem, we evaluated SSR161421, a new selective human adenosine A3 receptor antagonist, adenosine A3 receptor involvement in the antigen-induced responses in allergic sheep both in-vitro and in-vivo. Sheep were chosen for a number of reasons including: a) the sheep adenosine A3 receptor shows relatively high, i.e., 85%, overall identity with human A3 receptor [13]; b), allergic sheep like asthmatic patients react to inhaled adenosine with bronchoconstriction; and c) the extensive use of this model in evaluating compounds for the treatment of asthma and/or COPD [23]. The sheep used in this study were naturally sensitive to Ascaris suum antigen and were shown to develop both early and late antigen-induced responses to allergen provocation as well as antige-induced airway hyperresponsiveness- all important aspects of testing clinical candidate drugs [23].
As sheep are naturally sensitive to Ascaris suum, the response to antigen challenge both in-vitro and in-vivo is variable, reflecting well the variability of the antigen response in patients. As previously reported, this variability is more apparent in the early as compared to the late response [24].
Antigen challenge of sheep tracheal preparations, in-vitro, reflect the early airway response and is a useful tool to test the potential anti allergic actions of compounds. We used this system to select an appropriate antigen dose to elicit a moderate antigen contractile response (approx 12%) and then show that 10-6 M of the relatively selective adenosine A3 receptor agonist [25]. AB-MECA was able to significantly enhance this antigen- induced tracheal contraction. Similar studies were performed in isolated guinea pig tracheal preparations [12]. The ability of AB-MECA to enhance this response suggested that the adenosine A3 receptor was active in the antigen response. Therefore, we predicted that the selective A3 antagonist SSR161421 should inhibit this increased tracheal contraction. As expected, SSR161421 reversed the enhanced antigen reaction in a dose-dependent manner [26-29].
We then explored the potential role of A3 receptor activation in-vivo by treating allergic sheep with SSR161421. Following intravenous administration SSR161421 dose-dependently inhibited the late response and the subsequent airway hyper responsiveness. Because the late response and the post antigen-induced airway hyper responsiveness are physiological signals of airway inflammation [23] these findings would suggest that adenosine A3 receptor activation may be an important contributor to the inflammation resulting from antigen challenge. These effects were dose dependent as the 0.3 mg/kg dose had no effect on any endpoint. This is important as well as it rules out non-specific effects of the drug. It was also interesting to see that at the highest dose (3.0 mg/kg) SSR161421 showed a tendency to decrease the early response as well. This would be consistent with the blockade of the antigen-induced enhanced tracheal response in-vitro.
Conclusion
In summary, we have shown that the adenosine A3 receptor antagonist SSR161421 modifies allergen-induced responses in allergic sheep both in-vitro and in-vivo. These data support a role for A3 receptor stimulation in allergic asthma, and provide preliminary evidence that SSR161421 may have therapeutic potential.
References
- Hua X, Erikson CJ, Chason KD, Rosebrock CN, Deshpande DA, et al. (2007) Involvement of A1 adenosine receptors and neural pathways in adenosine-induced bronchoconstriction in mice. Am J Physiol Lung Cell Mol Physiol 293: L25-L32. [Crossref]
- Keir S, Boswell-Smith V, Spina D, Page C (2006) Mechanism of adenosine-induced airways obstruction in allergic guinea pigs. Br J Pharmacol 147: 720-728. [Crossref]
- Livingston M, Heaney LG, Ennis M (2004) Adenosine, inflammation and asthma-a review. Inflamm Res 53: 171-178. [Crossref]
- Polosa R (2002) Adenosine-receptor subtypes: their relevance to adenosine-mediated responses in asthma and chronic obstructive pulmonary disease. Eur Respir J 20: 488-496.
- Driver AG, Kukoly CA, Ali S, Mustafa SJ (1993) Adenosine in bronchoalveolar lavage fluid in asthma. Am Rev Respir Dis 148: 91-97. [Crossref]
- Meade CJ, Dumont I, Worrall L (2001) Why do asthmatic subjects respond so strongly to inhaled adenosine? Life Sci 69: 1225-1240. [Crossref]
- Hannon JP, Pfannkuche HJ, Fozard JR (1995) A role for mast cells in adenosine A3 receptor-mediated hypotension in the rat. Br J Pharmacol 115: 945-952. [Crossref]
- Fozard JR, Pfannkuche HJ, Schuurman HJ (1996) Mast cell degranulation following adenosine A3 receptor activation in rats. Eur J Pharmacol 298: 293-297. [Crossref]
- Feoktistov I, Polosa R, Holgate ST, Biaggioni I (1998) Adenosine A2B receptors: a novel therapeutic target in asthma? Trends Pharmacol Sci 19: 148-153. [Crossref]
- van den Berge M, Kerstjens HA, Postma DS (2002) Provocation with adenosine 5'-monophosphate as a marker of inflammation in asthma, allergic rhinitis and chronic obstructive pulmonary disease. Clin Exp Allergy 32: 824-830. [Crossref]
- Mikus EG, Boer K, Tímári G, Urbán Szabó K, Kapui Z, et al. (2013a) Interaction of SSR161421, a novel specific adenosine A3 receptor antagonist with adenosine A3 receptor agonists both in vitro and in-vivo. Eur J Pharmacol 699: 62-66.
- Mikus EG, Szeredi J, Boer K, Tímári G, Finet M, et al. (2013b) Evaluation of SSR161421, a novel orally active adenosine A3 receptor antagonist on pharmacology models. Eur J Pharmacol 699: 172-179. [Crossref]
- Salvatore CA, Jacobson MA, Taylor HE, Linden J, Johnson RG (1993) Molecular cloning and characterization of the human A3 adenosine receptor. Proc Natl Acad Sci USA 90: 10365-10369. [Crossref]
- Abraham WM, Stevenson JS, Chapman GA, Tallent MW, Jackowski J (1987) The effect of nedocromil sodium and cromolyn sodium on antigen-induced responses in allergic sheep in vivo and in vitro. Chest 92: 913-917. [Crossref]
- Abraham WM, Sielczak MW, Ahmed A, Cortes A, Lauredo IT, et al. (1994) Alpha4-integrins mediate antigen-induced late bronchial responses and prolonged airway hyperresponsiveness in sheep. J Clin Invest 93: 776-87.
- Crimi N, Palermo F, Oliveri R, Palermo B, Polosa R, et al. (1992) Protection of nedocromil sodium on bronchoconstriction induced by inhaled neurokinin A (NKA) in asthmatic patients. Clin Exp Allergy 22: 75-81. [Crossref]
- Mann JS, Cushley MJ, Holgate ST (1985) Adenosine-induced bronchoconstriction in asthma. Role of parasympathetic stimulation and adrenergic inhibition. Am Rev Respir Dis 132: 1-6. [Crossref]
- Polosa R, Phillips GD, Rajakulasingam K, Holgate ST (1991) The effect of inhaled ipratropium bromide alone and in combination with oral terfenadine on bronchoconstriction provoked by adenosine 5 monophosphate and histamine in asthma. J. Allergy Clin Immunol. 87: 939-947.
- Brown RA, Clarke GW, Ledbetter CL, Hurle MJ, Denyer JC, et al. (2008) Elevated expression of adenosine A1 receptor in bronchial biopsy specimens from asthmatic subjects. Eur Respir J 31: 311-319.
- Ethier MF, Madison JM (2006) Adenosine A1 receptors mediate mobilization of calcium in human bronchial smooth muscle cells. Am J Respir Cell Mol Biol 35: 496-502. [Crossref]
- Cicala C, Ialenti A (2013) Adenosine signaling in airways: toward a promising antiasthmatic approach. Eur J Pharmacol 714: 522-525. [Crossref]
- Calzetta L, Spina D, Cazzola M, Page CP, Facciolo F, et al. (2011) Pharmacological characterization of adenosine receptors on isolated human bronchi. Am J Respir Cell Mol Biol 45: 1222-1231. [Crossref]
- Abraham WM (2008) Modeling of asthma, COPD and cystic fibrosis in sheep. Pulm Pharmacol Ther 21: 743-754. [Crossref]
- Abraham WM (1995) Animal models of late bronchial responses. Eur Respir Rev 29: 211-217.
- Varani K, Cacciari B, Baraldi PG, Dionisotti S, Ongini E, et al. (1998) Binding affinity of adenosine receptor agonists and antagonists at human cloned A3 adenosine receptors. Life Sci 63: PL 81-87. [Crossref]
- Blackburn MR (2003) Too much of a good thing: adenosine overload in adenosine-deaminase-deficient mice. Trends Pharmacol Sci 24: 66-70. [Crossref]
- Blackburn MR, Datta SK, Kellems RE (1998) Adenosine deaminase-deficient mice generated using a two-stage genetic engineering strategy exhibit a combined immunodeficiency. J Biol Chem. 273: 5093-5100.
- el-Hashim A, D'Agostino B, Matera MG, Page C (1996) Characterization of adenosine receptors involved in adenosine-induced bronchoconstriction in allergic rabbits. Br J Pharmacol 119: 1262-1268. [Crossref]
- Baraldi PG, Cacciari B, Spalluto G, Bergonzoni M, Dionisotti S, et al. (1998) Design, synthesis, and biological evaluation of a second generation of pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidines as potent and selective A2A adenosine receptor antagonists. J Med Chem 41: 2126-2133. [Crossref]





