Non-alcoholic fatty liver disease (NAFLD) is characterized by the accumulation of lipids caused by an imbalance among synthesis, ingestion, oxidation and exporting fatty acids. Animal models using diets with high levels of lipids or carbohydrates and excessive treatment with glucocorticoids have mimicked the alterations in hepatic steatosis. Nutritional interventions using n-3 polyunsaturated fatty acids (EPA-eicosapentaenoic acid and DHA-docosahexaenoic acid) have shown to be able to decrease hepatic steatosis, particularly by reducing the activity of lipogenic enzymes and increasing β-oxidation flux. In this field, new mechanisms such as oxidative enzymes activity and changes in endocannabinoid system tone have been investigated to elucidate these effects. In addition, investigation of new EPA and DHA specific molecular targets have been discussed by new studies.
hepatic steatosis, liver, metabolism, n-3 fatty acids, pre-clinical models
Non-alcoholic fatty liver disease (NAFLD) is one of the most common forms of chronic liver disease in developed countries, affecting 20 to 30% of the general population [1-3]. This disease is defined by the pathological accumulation of excessive fat in the liver without alcohol consumption, like the accumulation of hepatic triglycerides (TGs) resulting from unbalanced uptake, synthesis, exportation and oxidation of fatty acids [4,5]. Such state is characterized by hepatic steatosis, liver cell injury, and lobular hepatitis [4].
Obesity is an important risk factor for the development of NAFLD, mainly visceral fat accumulation, therefore, insulin resistance may also be responsible for the development of NAFLD even in non-obese and lean individuals [6-8]. However, the pathogenesis of NAFLD is not completely clear. Multiple mechanisms, such as aberrant lipid metabolism, dysregulated cytokine production, oxidative stress, and inflammation in hepatocytes, are believed to be involved [9-12].
Other factors that play a role in hepatic lipid content can include diet, de novo hepatic lipid synthesis, and genetics factors [13-16]. Following such line of thinking, to reproduce the etiology, development, progression and outcome of liver disease, animal-based experimental models are commonly used, such as high caloric diet intake (overfeeding), high fat intake (especially saturated fatty acids), high intake of simple sugars and cafeteria diet [17-21]. Furthermore, some pharmacological interventions can also cause NAFLD. Synthetic glucocorticoids (GCs) are substances mimicking endogenous steroid hormones secreted by the adrenal cortex upon activation of the hypothalamic-pituitary-adrenal (HPA)-axis. They can contribute to development of metabolic syndrome. Synthetic GCs are commonly applied as anti-inflammatory drugs. However, the use for prolonged time or in high doses can cause side effects, such as weight gain, insulin resistance, hypertriglyceridemia, hyperphagia and central obesity [22-26].
Although the pathogenic mechanisms involved in hepatic lipid accumulation caused by diet or synthetic GC use are not completely understood, some studies have been conducted to find adjuvant strategies to attenuate the changes evidenced in this metabolic disorder, as the ingestion of polyunsaturated fatty acids (PUFAs) [27-30].
Fatty acids can influence many cell properties, resulting in altered metabolism, gene expression, modified responsiveness to hormones, and production patterns of biologically active substances. Therefore, fatty acids can modulate physiological functions and potentially beneficial to promote health and well-being [31].
In addition, some evidence suggests that PUFAs omega-3 (n-3), mainly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), can contribute to improve several metabolic dysfunctions (e.g. increase glucose tolerance, insulin sensitivity, and reducing the risk factors for chronic non-communicable diseases and metabolic syndrome) [32-39].
The effects of polyunsaturated fatty acids ingestion reducing accumulation of liver lipids in pre-clinical models have been shown by different research groups [40-44]. However, the molecular pathways explaining such effects are still under investigation. Among different pathways and molecular targets, the activation of the transcription factor peroxisome proliferator activated receptor-α (PPAR-α) is the most studied and most concise result [32,46-49]. These studies show that the activation of the PPAR leads to a lower expression and activity of lipogenic genes and enzymes, such as sterol regulatory element-binding protein 1c (SREBP-1c), carbohydrate responsive element-binding protein (ChREBP), fatty acid synthase (FAS), acetyl-CoA carboxylase (ACC), and HMG-CoA reductase. Also, PPAR activity and expression can be linked to increasing lipoprotein lipase (LPL) activity, carnitine palmitoyltransferase (CPT) activity, and β-oxidation flux, promoting higher oxidation of fatty acids [32,45-50]. Mice supplemented with fish oil (a source of EPA and DHA) and fed with high-fat diet have shown that increasing the expression of enzymes related to lipogenesis, and enzymes involved in the oxidative capacity are important for controlling the accumulation of liver lipids [51]. This study showed an increase in the activity of medium-chain acyl-CoA dehydrogenase (MCAD), acyl-CoA oxidase (AOX), and increased content of the peroxisomal membrane protein 70 (PMP70) in mice fed with fish oil [51]. Such data indicate that supplementation of fish oil reduces accumulation of the fatty acids in liver promoting increased oxidative capacity.
Furthermore, a recent study showed that purified EPA and DHA have different effects on atherogenic high fat (AHF) NAFLD development of diet-induced disease in mice. EPA and DHA reduced the SREBP-1 protein and expression of lipogenic genes. However, EPA was more effective than DHA in reducing mRNA expression of FAS, (ELOVL family member 6) Elovl6 and glycerol-3-phosphate acyltransferase (GPAT-1). The authors also showed that cell expression death inducing DFFA like effector c (CIDEC), a protein located in lipid droplets playing a key role in fatty liver formation, was significantly suppressed in the AHF + EPA, but not in the AHF + DHA group [52]. Therefore, it is important to consider that these PUFAs (EPA and DHA) have specific targets but can contribute together to attenuate the accumulation of fatty acids in the liver.
A study with LDLR -/- mice with Nonalcoholic Steatohepatitis induced by Western diet (WD) showed that diets containing EPA and DHA have a hepatoprotective effect [53]. The authors showed that dietary EPA and DHA attenuates hepatic inflammation by suppressing saturated (SFA), monounsaturated fatty acids (MUFA), and sphingomyelin production. Apparently, this was achieved by suppressing substrate availability (citrate) and the expression of enzymes (FAS, ATP citrate lyase-ACL, stearoyl-CoA desaturase-1- SCD1, serine-palmitoyl transferase long chain base subunit-1-SPTLC1, phosphatidylcholine: ceramide choline phosphotransferase 1-SGMS1) involved in these pathways. Furthermore, DHA and EPA seemed to control cellular levels of antioxidants such as ascorbate and α-tocopherol, and increased the formation of oxidized lipids that can be hepatoprotective (epoxy and di-hydroxy-fatty derivatives of EPA and DHA). In particular, this study showed that DHA improved methylglyoxal detoxification induced by WD, this finding was important to understand how DHA regulates glucose and lipid metabolism [53]. Thus, a diet with DHA>EPA reduced the progression of hepatic steatosis by controlling the activation of transcription factors involved in lipid metabolism, oxidative stress, and inflammation. This shows that, besides acting together in some experimental models, DHA and EPA can modulate some molecular pathways and the respective outcomes differently.
Additional mechanisms have been investigated to further elucidate the effects of EPA and DHA on hepatic lipid metabolism. Mice fed a WD containing cod (a fish source of EPA and DHA) showed a significant increase in the concentrations of EPA and DHA, and an attenuation in hepatic fat accumulation, accompanied by a change in the endocannabinoid system tone. The presence of higher concentrations of EPA and DHA, when compared to arachidonic acid, leads to the reduction of synthesis of 2-arachidonoylglycerol (2-AG), N-arachidonoylethanolamine (AEA), and increases the availability of substrate for the formation of endocannabinoid derivatives from EPA and DHA (e.g. eicosapentaenoyl ethanolamide (EPEA) and docosahexaenoyl ethanolamide (DHEA), respectively). This mechanism can partly explain the attenuation of the increase of hepatic lipids, and the development of obesity in WD and cod fed mice [54].
Thus, the most recent literature shows that EPA and DHA (n-3 PUFAs present in fish oil and oily fish) can attenuate the accumulation of lipids in the liver in pre-clinical models. Such effects are associated not only with n-3 fatty acids influencing the activity of proteins involved in lipogenesis and β-oxidation but also with metabolic oxidative enzymes and changes in endocannabinoid system tone (Figure 1). Further research should explore these mechanisms, especially distinct EPA and DHA effects on specific molecular targets. This could lead us to the next generation of effective therapeutic approaches.
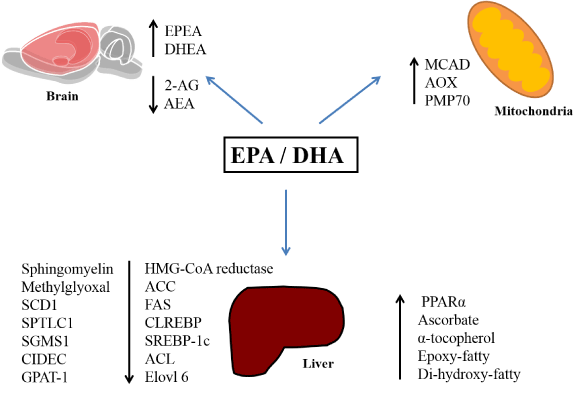
Figure 1. Summary of EPA and DHA molecular effects related to the reduction in hepatic lipid accumulation. Abbreviations: EPA: eicosapentaenoic acid, DHA: docosahexaenoic acid, EPEA: eicosapentaenoyl ethanolamide, DHEA: docosahexaenoyl ethanolamide, 2-AG: 2-Arachidonoylglycerol, AEA: N-arachidonoylethanolamine, MCAD: medium-chain acyl-CoA dehydrogenase, AOX: acyl-CoA oxidase, PMP70: peroxisomal membrane protein 70, SCD1: stearoyl-CoA desaturase-1, SPTLC1: serine-palmitoyl transferase long chain base subunit-1, SGMS1: phosphatidylcholine:ceramide choline phosphotransferase 1, CIDEC: death inducing DFFA like effector c, GPAT-1: glycerol-3-phosphate acyltransferase, ACC: acetyl-CoA carboxylase, FAS: fatty acid synthase, SREBP-1c: sterol regulatory element-binding protein 1c, ACL, Elovl 6: ELOVL family member 6, PPARα: peroxisome proliferator activated receptor-α, ChREBP: carbohydrate responsive element-binding protein
The authors confirm that there no conflict of interest.
The authors together contributed to the preparation of the article design, writing and critical review of the intellectual content of the article, as well as approval of the final version.
- Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, et al. (2012) The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, American College of Gastroenterology. and the American Gastroenterological Association. Hepatology 55: 1592-1609.
- Younossi ZM (2008) Review article: current management of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Aliment Pharmacol Ther 28: 2-12. [Crossref]
- Angulo P (2007) GI epidemiology: nonalcoholic fatty liver disease. Aliment Pharmacol Ther 25(8): 883-889.
- Clark JM, Diehl AM (2003) Defining nonalcoholic fatty liver disease: implications for epidemiologic studies. Gastroenterology 124: 248-250. [Crossref]
- Tamura S, Shimomura I (2005) Contribution of adipose tissue and de novo lipogenesis to nonalcoholic fatty liver disease. J Clin Invest 115: 1139-1142.
- Fan JG, Peng YD (2007) Metabolic syndrome and non-alcoholic fatty liver disease: Asian definitions and Asian studies. Hepatobiliary Pancreat Dis Int 6: 572-578. [Crossref]
- Cohen JC, Horton JD, Hobbs HH (2011) Human fatty liver disease: old questions and new insights. Science 332: 1519-1523. [Crossref]
- Takaki A, Kawai D, Yamamoto K (2013) Multiple Hits, Including Oxidative Stress, as Pathogenesis and Treatment Target in Non-Alcoholic Steatohepatitis (NASH). Int J Mol Sci 14: 20704-20728.
- Miller AM, Wang H, Bertola A, Park O, Horiguchi N, et al. (2011) Inflammation-associated interleukin-6/signal transducer and activator of transcription 3 activation ameliorates alcoholic and nonalcoholic fatty liver diseases in interleukin-10-deficient mice. Hepatology 54: 846-856. [Crossref]
- Wanless IR, Shiota K (2004) The pathogenesis of nonalcoholic steatohepatitis and other fatty liver diseases: a four-stepmodel including the role of lipid release and hepatic venular obstruction in the progression to cirrhosis. Semin Liver Dis 24: 99-106. [Crossref]
- Gupte P, Amarapurkar D, Agal S, Baijal R, Kulshrestha P, et al. (2004) Non-alcoholic steatohepatitis in type 2 diabetes mellitus. J Gastroenterol Hepatol 19: 854-858. [Crossref]
- Schwimmer JB, Deutsch R, Rauch JB, Behling C, Newbury R, et al. (2003) Obesity, insulin resistance, and other clinicopathological correlates of pediatric nonalcoholic fatty liver disease. J Pediatr 143: 500-505. [Crossref]
- Takaki A, Kawai D, Yamamoto K (2014) Molecular mechanisms and new treatment strategies for non-alcoholic steatohepatitis (NASH). Int J Mol Sci 15: 7352-7379. [Crossref]
- Nakamuta M, Kohjima M, Morizono S, Kotoh K, Yoshimoto T, et al. (2005) Evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease. Int J Mol Med 16: 631-635. [Crossref]
- Reddy JK, Rao MS (2006) Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am J Physiol Gastrointest Liver Physiol 290: 852-858. [Crossref]
- Kohjima M, Enjoji M, Higuchi N, Kato M, Kotoh K, et al. (2007) Re-evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease. Int J Mol Med 20: 351-358. [Crossref]
- Basaranoglu M, Basaranoglu G, Sabuncu T, Sentürk H (2013) Fructose as a key player in the development of fatty liver disease. World J Gastroenterol 19: 1166-1172. [Crossref]
- Vos MB, Lavine JE (2013) Dietary fructose in nonalcoholic fatty liver disease. Hepatology 57: 2525-2531. [Crossref]
- Tappy L, Lê KA (2012) Does fructose consumption contribute to non-alcoholic fatty liver disease? Clin Res Hepatol Gastroenterol 36: 554-560. [Crossref]
- Gao M, Ma Y, Liu D (2015) High-fat diet-induced adiposity, adipose inflammation, hepatic steatosis and hyperinsulinemia in outbred CD-1 mice. PLoS One 10: e0119784. [Crossref]
- Sampey BP, Vanhoose AM, Winfield HM, Freemerman AJ, Muehlbauer MJ, et al. (2011) Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: comparison to high-fat diet. Obesity (Silver Spring) 19: 1109-1117. [Crossref]
- Zhou J, Cidlowski JA (2005) The human glucocorticoid receptor: one gene, multiple proteins and diverse responses. Steroids 70: 407-417. [Crossref]
- Andrews RC, Wal2021 Copyright OAT. All rights reservulin resistance: old hormones, new targets. Clin Sci (Lond) 96: 513-523. [Crossref]
- Macfarlane DP, Forbes S, Walker BR (2008) Glucocorticoids and fatty acid metabolism in humans: fuelling fat redistribution in the metabolic syndrome. J Endocrinol 197: 189-204. [Crossref]
- Auvinen HE, Coomans CP, Boon MR, Romijn JA, Biermasz NR, et al. (2013) Glucocorticoid excess induces long-lasting changes in body composition in male C57Bl/6J mice only with high-fat diet. Physiol Rep 1: e00103. [Crossref]
- Barbosa AM, Francisco Pde C, Motta K, Chagas TR, Dos Santos C, et al. (2016) Fish oil supplementation attenuates changes in plasma lipids caused by dexamethasone treatment in rats. Appl Physiol Nutr Metab 41: 382-390. [Crossref]
- Assy N (2011) Nutritional recommendations for patients with non-alcoholic fatty liver diseases. World J Gastroenterol 17: 3375-3376. [Crossref]
- González-Périz A, Horrillo R, Ferré N, Gronert K, Dong B, et al. (2009) Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J 23: 1946-1957. [Crossref]
- Alexander-Aguilera A, Berruezo S, Hernández-Diaz G, Angulo O, Oliart-Ros R, et al. (2011) Dietary n-3 polyunsaturated fatty acids modify fatty acid composition in hepatic and abdominal adipose tissue of sucrose-induced obese rats. J Physiol Biochem 67: 595-604. [Crossref]
- Perez-Martinez P, Perez-Jimenez F, Lopez-Miranda J (2010) n-3 PUFA and lipotoxicity. Biochim Biophys Acta 1801: 362-366. [Crossref]
- Calder PC (2015) Functional Roles of Fatty Acids and Their Effects on Human Health. JPEN J Parenter Enteral Nutr 39: 18S-32S. [Crossref]
- Poudyal H, Panchal SK, Diwan V, Brown L (2011) Omega-3 fatty acids and metabolic syndrome: effects and emerging mechanisms of action. Prog Lipid Res 50: 372-387. [Crossref]
- Valenzuela R, Videla LA (2011) The importance of the long-chain polyunsaturated fatty acid n-6/n-3 ratio in development of non-alcoholic fatty liver associated with obesity. Food Funct 2: 644-648. [Crossref]
- Uauy R, Mena P, Rojas C (2000) Essential fatty acids in early life: structural and functional role. Proc Nutr Soc 59: 3-15. [Crossref]
- Serhan CN, Petasis NA (2011) Resolvins and protectins in inflammation resolution. Chem Rev 111: 5922-5943. [Crossref]
- Storlien LH, Baur LA, Kriketos AD, Pan DA, Cooney GJ, et al. (1996) Dietary fats and insulin action. Diabetologia 39: 621-631. [Crossref]
- Kris-Etherton PM, Harris WS, Appel LJ; American Heart Association. Nutrition Committee (2002) Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation,106(21), pp.2747-2257.
- Sirtori CR, Galli C (2002) N-3 fatty acids and diabetes. Biomed Pharmacother 56: 397-406. [Crossref]
- Hirabara SM, Folador A, Fiamoncini J, Lambertucci RH, Rodrigues CF Jr, et al. (2013) Fish oil supplementation for two generations increases insulin sensitivity in rats. J Nutr Biochem 24: 1136-1145. [Crossref]
- Popescu LA, Vîrgolici B, Lixandru D, Miricescu D, Condruţ E, et al. (2013) Effect of diet and omega-3 fatty acids in NAFLD. Rom J Morphol Embryol 54: 785-790. [Crossref]
- Bargut TC, Frantz ED, Mandarim-de-Lacerda CA, Aguila MB (2014) Effects of a diet rich in n-3 polyunsaturated fatty acids on hepatic lipogenesis and beta-oxidation in mice. Lipids 49: 431-444. [Crossref]
- Taltavull N, Muñoz-Cortés M, Lluís L, Jové M, Fortuño A, et al. (2014) Eicosapentaenoic acid/docosahexaenoic acid 1:1 ratio improves histological alterations in obese rats with metabolic syndrome. Lipids Health Dis 11: 31. [Crossref]
- de Castro GS, Cardoso JF, Calder PC, Jordão AA, et al. (2015) Fish oil decreases hepatic lipogenic genes in rats fasted and refed on a high fructose diet. Nutrients 7: 1644-1656. [Crossref]
- Pengfei Xu, Huan Wang, Abudurexiti Kayoumu, Mengyu Wang, Wei Huang, et al. (2015) Diet rich in Docosahexaenoic Acid/ Eicosapentaenoic Acid robustly ameliorates hepatic steatosis and insulin resistance in seipin deficient lipodystrophy mice. Nutr Metab (Lond) 12: 58, [Crossref]
- Zúñiga J, Cancino M, Medina F, Varela P, Vargas R, et al. (2011) N-3 PUFA supplementation triggers PPAR-a activation and PPAR-α/NF-κB interaction: anti-inflammatory implications in liver ischemia-reperfusion injury. PLoS One, 6: e28502. [Crossref]
- Liu X, Xue Y, Liu C, Lou Q, Wang J, et al. (2013) Eicosapentaenoic acid-enriched phospholipid ameliorates insulin resistance and lipid metabolism in diet-induced-obese mice. Lipids Health Dis 23(12): 109. [Crossref]
- Soni NK, Nookaew I, Sandberg AS, Gabrielsson BG (2015) Eicosapentaenoic and docosahexaenoic acid-enriched high fat diet delays the development of fatty liver in mice. Lipids Health Dis 14: 74. [Crossref]
- Konuma K, Itoh M, Suganami T, Kanai S, Nakagawa N, et al. (2015) Eicosapentaenoic Acid Ameliorates Non-Alcoholic Steatohepatitis in a Novel Mouse Model Using Melanocortin 4 Receptor- Deficient Mice. PLoS one, 10: e0121528. [Crossref]
- Su J, Ma C, Liu C, Gao C, Nie R, et al. (2016) Hypolipidemic Activity of Peony Seed Oil Rich in α -Linolenic, is Mediated Through Inhibition of Lipogenesis and Upregulation of Fatty Acid β -Oxidation. J Food Sci 81: H 1001-1009. [Crossref]
- Anderson N, Borlak J (2008) Molecular mechanisms and therapeutic targets in steatosis and steatohepatitis. Pharmacol Rev 60: 311-357. [Crossref]
- Liu M, Montgomery MK, Fiveash CE, Osborne B, Cooney GJ, et al. (2014) PPARa-independent actions of omega-3 PUFAs contribute to their beneficial effects on adiposity and glucose homeostasis. Sci Rep 2: 5538. [Crossref]
- Suzuki-Kemuriyama N, Matsuzaka T, Kuba M, Ohno H, Han S, et al. (2016) Different Effects of Eicosapentaenoic and Docosahexaenoic Acids on Atherogenic High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease in Mice. PLoS One 11: e0157580. [Crossref]
- Depner CM, Philbrick KA, Jump DB (2013) Docosahexaenoic acid attenuates hepatic inflammation, oxidative stress, and fibrosis without decreasing hepatosteatosis in a Ldlr (-/-) mouse model of western diet-induced nonalcoholic steatohepatitis. J Nutr. 143: 315-23. [Crossref]
- Liisberg U, Fauske KR, Kuda O, Fjære E, Myrmel LS, et al. (2016) Jan Kopeckyb, Lise Madsena. Intake of a Western diet containing cod instead of pork alters fatty acid composition in tissue phospholipids and attenuates obesity and hepatic lipid accumulation in mice. J Nutr Biochem 33: 119-127. [Crossref]

